ORIGINAL ARTICLE
Description of the epidemiology of healthcare-associated infections in the military hospital of Tunis
Aloui Ghaith1*, Yousfi Mohamed Ali1,2 and Bennour Sana1
1Department of Pharmacy, Main Military Training Hospital of Tunis, Tunis, Tunisia; 2Faculty of Pharmacy of Monastir, Monastir, Tunisia
Abstract
Background: Healthcare-associated infections (HAIs) are the most common adverse events in healthcare delivery and the most frequently reported worldwide. Understanding its epidemiology can help stratify the problems and effectively determine prevention and control strategies.
Objective: The aim of this study is to describe the epidemiology of HAIs in the military hospital of Tunis.
Design: This is a retrospective descriptive study covering the year 2021. It concerns patients who stayed more than 48 h and who developed an infection meeting the criteria of HAIs.
Results: A total of 380 HAIs were detected. This represents 2.49 per 1,000 days of hospitalization and 8% of admissions. Men were more affected than women. The anesthesia-intensive care department was the most involved (32.25%). Diabetes mellitus was the most encountered comorbidity (27%). The pulmonary system was the most affected site (46.8%). Germs were identified in 26% of cases. Klebsiella pneumoniae was the most incriminated germ (31%), followed by Staphylococcus aureus (26%). Vancomycin was the most prescribed broad-spectrum antibiotic (24%).
Conclusions: Epidemiological surveillance is an essential tool to evaluate the impact of infection prevention and control policies. It would be desirable to reinforce the global policy in our hospital, which is mainly based on the respect of hygiene rules by the staff.
Keywords: epidemiology; healthcare-associated infections; hygiene; infection prevention and control
Citation: Int J Infect Control 2024, 20: 23765 – http://dx.doi.org/10.3396/ijic.v20.23765
Copyright: © 2024 Aloui Ghaith et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 10 September 2023; Accepted: 5 February 2024; Published: 4 April 2024
Competing interests and funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
*Aloui Ghaith, Department of Pharmacy, Main Military Training Hospital of Tunis, Montfleury; 1008 Tunis, Tunisia. Phone: +21692426403. Email: ghaith.aloui@fphm.u-monastir.tn
Healthcare-associated infections (HAIs) are the most common adverse events in caregiving and most frequently reported worldwide (1). The occurrence of HAIs can be life-threatening for patients and sets an additional economic burden on healthcare facilities that are already struggling.
The French society of hospital hygiene defines HAIs as an infection that occurs during or after the management (diagnostic, therapeutic, palliative, preventive, or educational) of a patient, and that was neither present nor incubating at the beginning of the care (2).
If the infectious status of the patient at admission is unknown, the infection is generally considered to be associated with care if it appears after a delay of at least 48 h of hospitalization or a delay longer than the incubation period of the infection. And in case of surgical site infection, the commonly accepted period is 30 days, or, if a prosthesis or implant is placed, 1 year after the procedure (3).
Understanding the epidemiology of HAIs can help stratify problems and effectively determine prevention and control strategies (4).
Knowing the chain of infection, especially the modes of transmission, can go a long way in helping healthcare workers prevent these infections (5).
This is why the study of HAIs at the main military training hospital of Tunis is the first element of understanding their occurrence, distribution, and severity.
Our hospital has a total of 609 beds in 29 different medical and surgical departments. During the year 2021, we recorded 4,750 admissions, with a cumulative of 152,442 days of hospitalization (DH). The prevention and control of HAIs in our hospital involves the infection control committee (ICC), the hospital hygiene, and the pharmacy departments.
The aim of this work is to measure the incidence of HAIs per 1,000 hospital days and per 100 admissions and to describe the epidemiology of HAIs at the hospital during the year 2021.
Methods
This is a descriptive retrospective study that took place at the main military training hospital of Tunis over a 1-year period (2021).
The study population consists of patients admitted to the hospital in 2021, who stayed more than 48 h and developed an infection that met the criteria of HAIs.
We excluded patients who developed an HAI outside our hospital.
The declaration of HAIs in our hospital is conducted through a form, which contains general information about the patient (clinical department, sex, and comorbidities) and the characteristics of the infection (site, samples taken, microorganism if identified, and antibiotics prescribed).
The prescriber must attach a copy of the HAIs declaration form to the antibiotic prescriptions form, which is a part of our hospital’s antimicrobial stewardship policy. It concerns a dozen antibiotics chosen either because of their broad-spectrum (imipenem-cilastatin), or because of their high cost (linezolid), or by desire to control their consumption. These forms are validated by a pharmacist after verification of the antibiotic indication, the prescribed dosage, and the duration of treatment.
If the declaration form is not completed by the prescriber, the HAIs are considered unreported.
Data were collected through:
- HAIs declaration forms,
- Antibiotic prescription forms,
- Patients’ medical records,
- The hospital’s ‘SysLab’ software, which provides the results of microbiological exams.
The description of the epidemiology of HAIs was based on the incidence of HAIs and its distribution according to the characteristics of the affected population (sex, department, and comorbidities), the sites affected, the germs involved, and the antibiotics prescribed to treat the infection.
Results
During this study, we detected 380 HAIs, which represents 2.49 per 1,000 DH and 8% of hospital admissions in 2021.
Our study showed that men are more affected than women. The sex ratio of the study population was 1.4.
Figure 1 shows the distribution of HAIs across the hospital departments during the study.
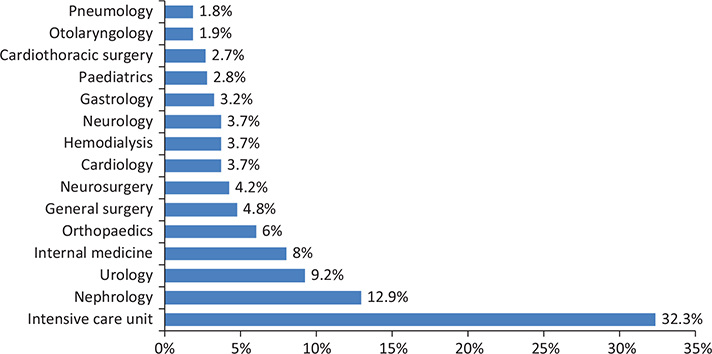
Fig. 1. Distribution of healthcare-associated infections in the hospital.
The anesthesia-intensive care unit was the most concerned by the incidence of HAIs, with 2.58% of admissions and 13.64 per 1,000 DH, followed by the nephrology department, with 1.03% of admissions and 9.95% per 1,000 DH.
Diabetes was the most common comorbidity in patients with HAIs in this study (27%), followed by renal failure (Figure 2).
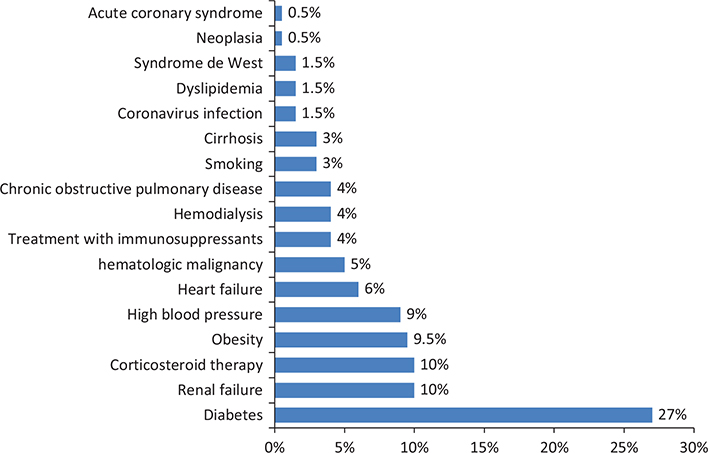
Fig. 2. Distribution of comorbidities in patients with healthcare-associated infections.
We determined that the lung was the most affected site, with 3.74% of admissions and 1.16 per 1,000 DH, followed by the urinary tract, with 2.3% of admissions and 0.72 per 1,000 DH (Figure 3).
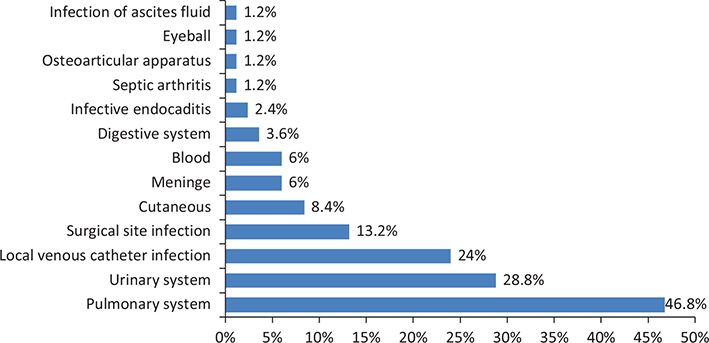
Fig. 3. Distribution of healthcare-associated infections by sites.
Microorganisms were identified in 26% of cases. We have presented in Table 1 the different germs isolated of samples from infected sites.
Klebsiella pneumoniae was the most implicated microorganism in HAIs (31%), followed by Staphylococcus aureus (26%), Escherichia coli (13%), and Pseudomonas aeruginosa (8%).
For HAIs affecting the pulmonary system, the most common germs were Klebsiella pneumoniae (32%), Acinetobacter baumanii (32%), and Pseudomonas aeruginosa (11%).
As for HAIs affecting the urinary tract, the most common germs were Klebsiella pneumoniae (43%), Escherichia coli (22%), and Acinetobacter baumanii (22%).
Vancomycin was the most prescribed antibiotic (24%) to treat HAIs followed by broad-spectrum beta-lactams: 22% imipenem-cilastatin and 15% piperacillin-tazobactam (Figure 4).
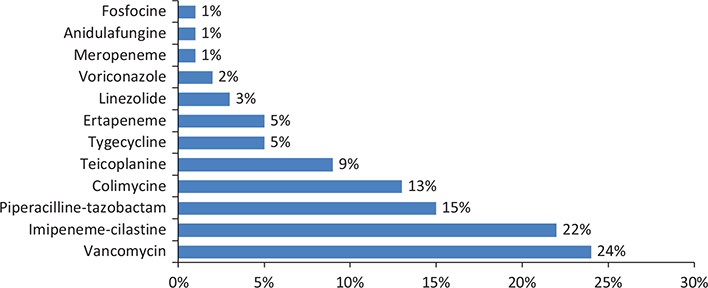
Fig. 4. Distribution of broad-spectrum anti-infectives prescribed for the treatment of healthcare-associated infections.
Vancomycin was prescribed primarily for the treatment of catheter-based infections (50%), surgical site infections (11.5%), and digestive infections (11.5%).
The percentage of HAIs reported by completing the dedicated reporting form was 57% (n = 217). We have seen a decrease in the number of HAIs reported throughout the year 2021 (Figure 5).
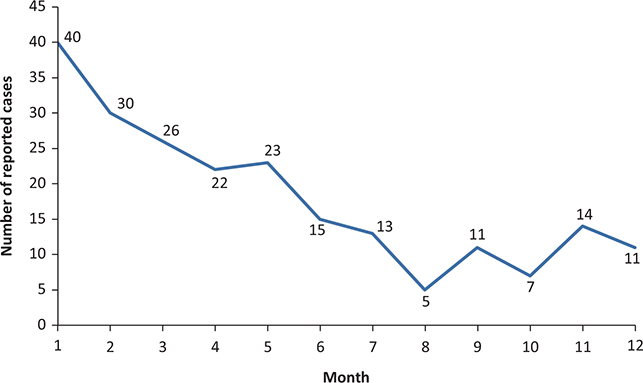
Fig. 5. Evolution of the number of healthcare-associated infections reported during 2021.
Discussion
HAIs affect millions of patients each year worldwide and result in significant morbidity, mortality, and funding costs for healthcare facilities (6).
In our study, the incidence of HAIs was 2.49 per 1,000 DH and 8 per 100 admissions.
An overall incidence of 5% is generally observed in French hospitals where incidence surveys are conducted (7).
In addition, Gaudichon et al. estimate that about 5% of patients will develop an infection during their hospitalization (8).
In Italy, the incidence rate was 6.7%, and in the United Kingdom, the estimated rate of HAIs was 8.2% (4).
We found that men seem to be more affected than women by HAIs. This is in line with what has been reported in literature (9–11).
The anesthesia-intensive care unit was the most affected department.
A study conducted by Trifi et al. in a Tunisian anesthesia-intensive care unit showed that the incidence was 66.4 HAI per 1,000 DH with pulmonary infections at the top of the list. The authors of this study concluded that it is becoming acceptable that the frequency of HAIs in intensive care units is higher than in other care sectors (12). Thus, the patient in intensive care has a preexisting fragility due to his medical history. This initial vulnerability can be aggravated by diagnostic and therapeutic management with the performance of many invasive procedures. In addition, patients admitted to the intensive care unit tend to have longer hospital stays. Many other authors have confirmed these findings (10, 11, 13).
Diabetes mellitus was the most associated comorbidity in our study.
Pooled data from six articles included in a meta-analysis indicated that patients with diabetes mellitus were 2.32 times more likely to develop HAIs (14).
Other comorbidities were encountered in our study such as renal failure, corticosteroid therapy, and obesity.
Some risk factors that contribute to the infection are listed as extrinsic factors such as exposure to invasive medical devices (surgical procedures, mechanical ventilation in intensive care, puncture or infusion placement, dialysis, endoscopies, etc.) as well as the resident flora (12).
In less frequent cases, HAIs involve bacteria acquired through contact with other people (caregivers, other patients, family members, and visitors), due to a lack of compliance with hygiene rules, including hand hygiene.
During our study, the pulmonary system topped the list of infected sites followed by the urinary tract. These results are consistent with a meta-analysis (15), including 10 studies carried out in the greater Maghreb region. Thus, pneumonia was preponderant, and the most incriminated germs were Klebsiella pneumonia and Escherichia coli. Similar to our study, Klebsiella pneumonia was the most implicated germ in HAIs affecting the pulmonary system.
We should mention that by incorporating a specific indication for device-related HAIs in our reporting form, we could enhance the precision and utility of our surveillance system. This addition not only allows for a more accurate categorization of infections but also provides valuable insights into the context and potential causes of HAIs.
Our results indicate that vancomycin was the main antibiotic prescribed for the treatment of HAIs, followed by imipenem-cilastatin and piperacillin-tazobactam. In most cases, antibiotics were prescribed in combination as part of empiric antibiotic therapy.
We have found that the HAIs incidence data are underestimated due to lack of reporting. Indeed, we need the information contained in the declaration form to improve the epidemiological surveillance of HAIs and to be able to put in place appropriate corrective and preventive measures.
It is essential to emphasize that the referring doctors play a pivotal role in the accuracy of the data, as they are responsible for completing both the prescription and reporting forms. Additionally, our study incorporates a feedback loop with referring doctors, facilitating communication and addressing any queries or concerns that may arise during the data validation process. This iterative approach contributes to the continuous improvement of data quality and enhances the overall reliability of our findings.
The integration of HAI declaration forms with antibiotic prescriptions is a strategic step in aligning infection reporting with antimicrobial stewardship. This not only streamlines the documentation process but also ensures a direct connection between the identified HAIs and the corresponding prescribed treatment. Involving pharmacists in the validation process adds an additional layer of expertise to the surveillance system. Their verification of antibiotic indication, prescribed dosage, and treatment duration helps ensure that the prescribed antibiotics align with established guidelines and best practices.
Several factors may contribute to the non-reporting of HAIs. Some may be related to the effect of the survey itself. Healthcare professionals may be aware of the ongoing study, and this may influence their reporting practices.
Another potential factor is the shortness of steam for physicians in the reporting process. Over time, physicians may experience some discomfort in regularly following up on HAIs reports, especially by filling out a form that contains both patient and infection details.
Further analysis is needed to understand the underlying reasons for the gradual decline in the number of reported HAIs.
Regular communication with prescribers, as well as ongoing training sessions, can contribute to continuous improvement in the surveillance system. This adaptability is essential for staying abreast of emerging challenges and incorporating best practices into the hospital’s antimicrobial stewardship and infection prevention and control policies.
It is essential to maintain the study of the incidence of HAIs in our institution on a regular basis each year in order to closely monitor the evolution of incidence rates and to be able to react quickly in the event of significant fluctuations.
A study conducted by Stempliuk et al. (16) that focuses on the oversight of HAIs in low- and middle-income countries has shown that HAIs are under-reported. In fact, they found that the availability of qualified personnel is limited in these countries, although these studies are essential for developing a prevention policy that is well targeted.
In many countries, the reporting system is based on paper or spreadsheets sent by e-mail. This leads to a delay in data collection and analysis and increases the risk of errors (16).
Conclusion
In conclusion, epidemiological surveillance of HAIs is an essential tool that allows us to assess the impact of current infection prevention and control policies.
It is imperative to ensure that prescribers provide the required information on each HAI by conscientiously completing the reporting form, in order to improve our understanding of the epidemiology.
The germs implicated in HAIs are multiresistant, which implies the reinforcement of hand hygiene rules, the isolation of patients, and possibly the need for periodic disinfection of departments when the incidence rate of HAIs reaches a certain limit.
It would be desirable to strengthen the overall policy of infection prevention and control in our hospital, which is mainly based on compliance with hygiene rules. This is based on the awareness about the importance of hand hygiene (use of hydroalcoholic solutions) as well as the implementation of an active strategy to control the spread of multiresistant germs by isolating colonized or infected patients and controlling the environment by strengthening cleaning and disinfection measures, especially at the level of intensive care units.
References
| 1. | MacLaurin A, Amaratunga K, Couris C, Frenette C, Galioto R, Hansen G, et al. Measuring and monitoring healthcare-associated infections: a Canadian collaboration to better understand the magnitude of the problem. Healthc Q 2020 Feb 3; 22(Suppl): 116–28. doi: 10.12927/hcq.2020.26040 |
| 2. | SF2H_surveiller-and-prevent-the-IAS-2010.pdf. Available from: https://sf2h.net/wp-content/uploads/2010/09/SF2H_surveiller-et-prevenir-les-IAS-2010.pdf [cited 20 November 2022]. |
| 3. | Gachot B, Coriat P. The medico-judicial risk of nosocomial infections. Med Right 2019; 2019(159): 137–41. doi: 10.1016/j.meddro.2019.04.001 |
| 4. | Unahalekhaka. Epidemiology of healthcare-associated infections. 2014. Available from: https://www.theific.org/wp-content/uploads/2014/08/Ch3French.pdf [cited 7 June 2023]. |
| 5. | Bouafia N, Chouchène I, Cheikh AB, Bouchoucha S, Njah M. Risk of death from infections associated with medical devices risk of mortality due to device associated infection. Tunis Med 2015; 93: 8. |
| 6. | Poirier E, Boulanger V, MacLaurin A, Quach C. National health care-associated infection surveillance programs: a scoping review. Can Commun Dis Rep 2022; 48(7/8): 373–84. doi: 10.14745/ccdr.v48i78a05 |
| 7. | Hall. Epidemiology of healthcare-associated infections. 2010. Available from: https://www.infectiologie.org.tn/pdf_ppt_docs/revues/1-2010/epidemiologie.pdf [cited 7 June 2023]. |
| 8. | Gaudichon A, Astagneau P. Infecciones nosocomiales e infecciones asociadas a la atención sanitaria. EMC Tratado Med 2022; 26(2): 1–8. doi: 10.1016/S1636-5410(22)46451-8 |
| 9. | Öncül O, Öksüz S, Acar A, Ülkür E, Turhan V, Uygur F, et al. Nosocomial infection characteristics in a burn intensive care unit: analysis of an eleven-year active surveillance. Burns J Int Soc Burn Inj 2014; 40(5): 835–41. doi: 10.1016/j.burns.2013.11.003 |
| 10. | Abdel-Fattah MM. Nosocomial pneumonia: risk factors, rates and trends. East Mediterr Health J 2008; 14(3): 546–55. |
| 11. | Chen Y, Zhao JY, Shan X, Han XL, Tian SG, Chen FY, et al. A point-prevalence survey of healthcare-associated infection in fifty-two Chinese hospitals. J Hosp Infect 2017; 95(1): 105–11. doi: 10.1016/j.jhin.2016.08.010 |
| 12. | Trifi A, Abdellatif S, Oueslati M, Zribi M, Daly F, Nasri R, et al. Nosocomial infections: state of play in a nosocomial intensive care unit infections: current situation in a resuscitation-unit. Tunis Med 2017; 95: 6. |
| 13. | Al-Asmary SM, Al-Helali NS, Abdel-Fattah MM, Al-Jabban TM, Al-Bamri AM. Nosocomial urinary tract infection. Risk factors, rates and trends. Saudi Med J 2004; 25(7): 895–900. doi: 10.1086/502336 |
| 14. | Teymourzadeh E, Bahadori M, Fattahi H, Rahdar HA, Mirzaei Moghadam S, Shokri A. Prevalence and predictive factors for nosocomial infection in the military hospitals: a systematic review and meta-analysis. Iran J Public Health 2021; 50(1): 58–68. doi: 10.18502/ijph.v50i1.5072 |
| 15. | Zoukal S, Tsoumbou-Bakana G, Traore B, Nani S, Hassoune S. Infections associated with neonatal care in the Greater Maghreb region. Systematic review and meta-analysis. Rev Epidemiol Public Health 2021; 69(2): 88–95. doi: 10.1016/j.respe.2021.01.007 |
| 16. | Stempliuk V. Surveillance of healthcare-associated infections in low- and middle-income countries: from the need to a reality. Curr Treat Options Infect Dis 2018; 10(1): 1–6. doi: 10.1007/s40506-018-0148-x |