ORIGINAL ARTICLE
Use of different designed needle-free connectors: a snapshot of central venous catheter intraluminal blood occlusion and central line–associated bloodstream infection in hospitals
Victor R. Lange*
AHMC Healthcare Inc., San Gabriel Valley, California, U.S.A.
Abstract
Background: Needle-free connectors (NCs), originally designed to improve the safety of healthcare workers, are increasingly being used to mitigate central line–associated bloodstream infection (CLABSI) and central venous catheter intraluminal blood occlusion (CVC-IBO) in patients. There are numerous NCs on the market, with varied internal and external designs and features.
Objectives: The purpose of this study was to compare the frequency, clinical, and financial impact of CVC-IBO and CLABSI among 16 California (USA) hospitals using differently designed NCs.
Method: Data were collected by sending a survey to the hospitals, which had varying bed capacities and patient populations that were committed to reducing CVC-IBO and CLABSI. In each hospital, CLABSI rates were tracked as defined by the National Healthcare Safety Network.
Results: Hospitals using the BD MaxPlus™ or MaxZero™ Needle-free Connector, the only device with a solid external access surface, were found to have a significantly lower CLABSI rate (1.32 vs. 2.95 per 1,000 central-line days [CLDs]; P = 0.0052) and CVC-IBO rate (1.51 vs. 4.04 per 1,000 CLDs; P = 0.0065) versus those using devices with a nonsolid access surface.
Conclusion: Hospitals using the MaxPlus™ or MaxZero™ NC also had significantly higher cost saving (per 100 patient days) associated with lower use of tissue plasminogen activator versus devices with a nonsolid access surface ($219 vs. $510 USD; P = 0.01). These results highlight the clinical importance of NC design components and their contributions to risk of CLABSI and catheter occlusion.
Keywords: Central line–associated bloodstream infection (CLABSI); Central venous catheter intraluminal blood occlusion (CVC-IBO); Needle-free connector (NC); Solid access surface.
Citation: Int J Infect Control 2024, 20: 23731 – http://dx.doi.org/10.3396/ijic.v20.23731
Copyright: © 2024 Victor R. Lange. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 2 August 2023; Revised: 2 October 2023; Accepted: 5 October 2023; Published: 9 August 2024
Competing interests and funding: There are no potential conflicts of interest. This study was independently developed and undertaken by the author. Technical writing support was funded by BD. There were no current funding sources for this study. BD supported technical writing assistance via FORCE Communications but had no additional role in study design, data collection and analysis, decision to publish, or manuscript preparation. BD provided financial assistance for writing and editorial services in the preparation of this manuscript.
*Victor R. Lange, AHMC Healthcare Inc., 438 West Las Tunas Drive, San Gabriel, CA 91776. Email: Victor.lange@ahmchealth.com
According to the US Centers for Disease Control and Prevention, approximately 385,000 needlestick and sharps-related injuries occur annually in hospital-based settings (1). These injuries increase the risk of exposure to bloodborne pathogens and are costly to both healthcare personnel and healthcare systems (1, 2). In the 1990s, needle-free connectors (NCs) were developed (3) to specifically reduce the risk of accidental sharps injuries (4). In recent years, more efficient and safe NCs (e.g. BD MaxPlus™ and BD MaxZero™ Needle-free Connectors) with unique design characteristics and capabilities that aim to reduce catheter-related occlusion (5) and bloodstream infection (6) have become available.
NC designs range from simple split-septum devices to more complex constructions containing multiple internal moving components (e.g. mechanical valves), each permitting needleless catheter access (7). NCs should minimize catheter occlusion risk and allow for easy and effective decontamination of the access surface, enabling healthcare workers to reduce needlestick injury and risk to patients (5). The inherent design characteristics of the NC determine its use and operation (5, 8). NCs are accessed by applying pressure from the male luer of a syringe or tubing (8). This applied pressure allows the cannula or male luer to open or depress the NC septum. Once the male luer enters in the NC, the fluid flows through a pathway determined by the NC (8). Fluid paths through NCs should minimize dead space, areas where fluid can be trapped and cannot be flushed or disinfected, and be visibly clear so that clinicians can assess their flush technique (7, 8). Once the fluid is flushed through the NC, the male luer is removed, and the subsequent fluid displacement at the tip of the catheter can be positive, negative, or neutral, depending on the NC design (Fig. 1). Positive fluid displacement causes a small amount of fluid to be expelled through the catheter tip, helping to prevent blood reflux into the catheter, whereas negative displacement allows a small amount of blood to reflux into the catheter (5). Although manufacturers define neutral fluid displacement as no fluid movement in either direction (5), minimal reflux still exists (9).
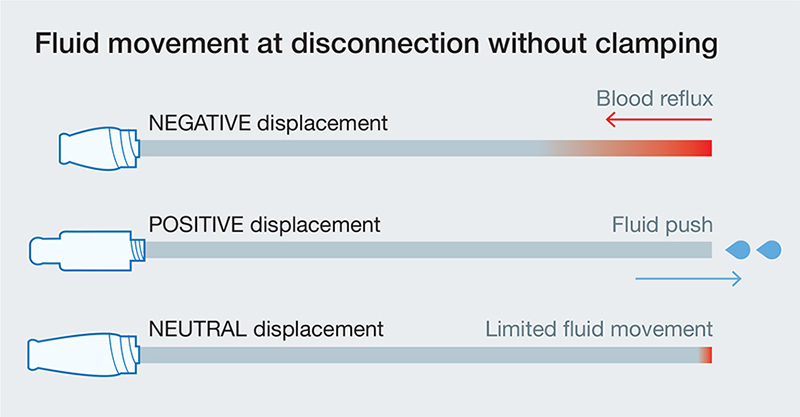
Fig. 1. Fluid flow in NFCs of various displacement type.
Previous studies have shown an increased risk of central line–associated bloodstream infection (CLABSI) when using NCs that have positive or negative displacement when compared to split-septum connectors (10). However, other studies have shown that positive-displacement connectors may not increase CLABSI risk (11, 12). It has also been suggested that CLABSI risk does not depend on displacement type but could be device specific and may depend more on the access-surface topography or the device technology (13).
The NC design may also increase occlusion risk. Some studies have shown that NCs using mechanical valves decreased catheter occlusion rates versus NCs using split-septum connectors (14). Other studies have focused on the displacement type contributing to the risk of occlusion. One showed no difference in occlusion rate between positive-displacement connectors and negative-displacement split-septum connectors (15), whereas a more recent study associated reduced occlusion risk with the use of a neutral-displacement device (16). Conclusive evidence on NC design and occlusion risk remains unknown given that these studies differed in design, occlusion type measured, patient population, sample size, and catheter care (14–16).
Should occlusion occur, use of tissue plasminogen activator (tPA), such as Alteplase, is safe and effective to clear the device (17). A bolus of 2 mg/2 mL is usually used to clear a catheter, but if the function is not restored after 120 min of dwell time, a second dose may be administered (18). Using specific NCs could reduce the risk of occlusion. A reduction in occlusions could reduce the need to purchase tPA, as well as treatment delay and nursing time spent managing occluded catheters, which would reduce cost.
In addition, CLABSI has a high cost burden, approximately $46,000 USD per case, with an annual infection rate of 250,000 (19). CLABSI leads to long hospital stays (19) and even death (20). According to surveillance data in 50 countries that are part of the International Nosocomial Infection Control Consortium, CLABSI rates in intensive care units (ICUs) in the United States are approximately 0.8 per 1,000 central-line days (CLDs) and globally are five-fold higher, with rates of 4.1 per 1,000 CLDs (19, 21). In 2019, general acute-care hospitals across the United States reported a standardized infection ratio of 0.69 (22).
The purpose of this study was to compare the incidence rates of central venous catheter intraluminal blood occlusion (CVC-IBO) in conjunction with CLABSI in 16 California (USA) hospitals and correlate them with differently designed NCs.
Methods
In 2017, a multicenter voluntary cross-sectional descriptive survey (Fig. 2) was conducted by using JotForm® (JotForm Inc.; San Francisco, California). Data were collected from Northern and Southern California hospitals that varied in type (e.g. acute care), bed size (e.g. <50 to >500), and patient population (e.g. patient-days). Facilities were alike in their desire to reduce CLABSI and CVC-IBO rates and in the interventions introduced.
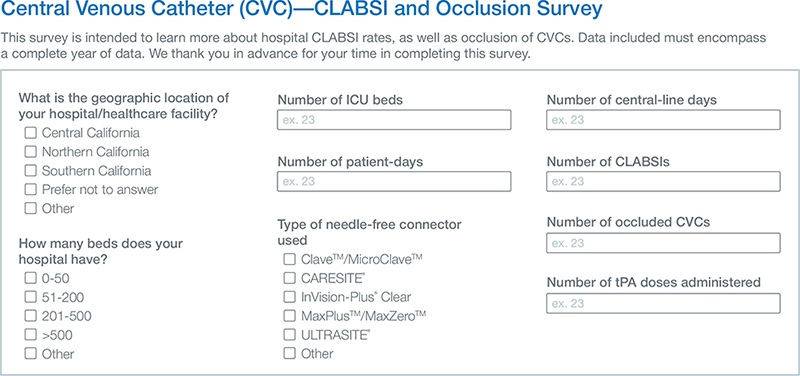
Fig. 2. Example of survey questions and interface.
Clinical outcomes assessed with the survey included the number of CLDs and patient-days, NC type, the number of CLABSIs and CVC-IBOs, and the number of tPA doses administered in a year. CVC occlusion is a complication, in which blood cannot be aspirated, but infusion through the catheter is possible or complete, or neither aspiration nor infusion is possible (23). CLABSI was tracked by all facilities, as defined by the National Healthcare Safety Network.
The CLABSI rate was calculated as the number of CLABSIs per 1,000 CLDs. The CVC-IBO rate was calculated as the number of CVC-IBOs per 1,000 CLDs. The tPA utilisation rate was calculated as the number of tPA doses per 100 patient-days. Cost per 100 patient-days was calculated by multiplying tPA utilisation rate by $110 (24). Annual cost was estimated by multiplying the total number of annual tPA doses per hospital by $110 (the estimated cost per tPA dose).
Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to perform the statistical analysis (ANOVA one-way analysis of variance). Data were represented as mean ± standard error of the mean (SEM), and statistical significance was set to a minimum of P < 0.05 for each comparison.
Results
Sixteen hospitals in Northern and Southern California responded to the survey and provided the details listed in Table 1. The mean number of beds was 313 (range, 50–698), and of ICU beds was 30 (range, 6–79). Each hospital used one of the following five varieties of NC:
- Clave™/MicroClave™ (ICU Medical),
- CARESITE® (B. Braun Medical Inc.),
- MaxPlus™/MaxZero™ (BD),
- InVision-Plus® Clear (RyMed Technologies),
- ULTRASITE® (B. Braun Medical Inc.).
Table 2 describes the features and characteristics of the five NC varieties used by the hospitals (8). NCs differed in design (e.g. smooth, flat, tightly sealed surface, or irregular) and characteristics of displacement type. Seven hospitals used NCs with a solid, flat, sealed top surface, while nine used NCs with irregular top surfaces (e.g. with space between the seal and inner NC diameter).
| Displacement | Design type | |
| CARESITE | Positive | Nonsolid access surface; split septum; luer access |
| Clave/MicroClave | Neutral | Nonsolid access surface; split septum; luer access; internal blunt cannula |
| MaxPlus/MaxZero | Positive | Solid access surface; luer access |
| InVision-Plus Clear | Neutral | Nonsolid access surface; luer access; septum; internal cannula |
| ULTRASITE | Positive | Nonsolid access surface; luer access; mechanical valve with internal spring |
| NA = not available; NFC = needle-free connector. | ||
The survey captured 88,151 patient-days and included 30,299 CLDs (Table 3) from 16 hospitals. The mean CLABSI rate of the 16 hospitals was 2.34 per 1,000 CLDs, while the mean CVC-IBO rate was 3.09. There was no correlation between CLDs and CLABSI or CVC-IBO rates in each hospital, nor between the number of patient-days and CLABSI or CVC-IBO rates.
The average CVC-IBO rate in hospitals using a solid-access-surface NC (MaxPlus™/MaxZero™) was 1.51 per 1,000 CLDs (Fig. 3), whereas the average rate in hospitals using nonsolid-access-surface NCs was 4.04 per 1,000 CLDs. The rate was significantly lower in hospitals using solid-access-surface NCs versus those using nonsolid-access-surface NCs (P = 0.0065).
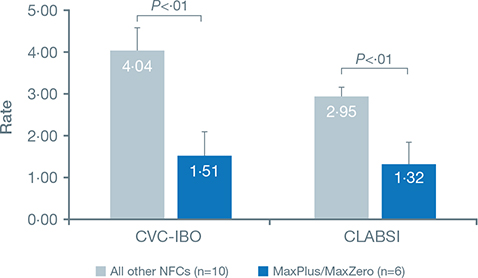
Fig. 3. CVC-IBO and CLABSI rates in hospitals using MaxPlus/MaxZero vs other reported NFCs.
The average CLABSI rate in hospitals using a solid-access-surface NC was significantly lower than that in hospitals using a nonsolid-access-surface NC (1.32 versus 2.95 per 1,000 CLDs; P = 0.0052).
The number of tPA doses used by hospitals grouped by NC used varied widely. On average, hospitals using MaxPlus™/MaxZero™ NCs (solid access surface) used 88 doses in a year, whereas hospitals using other NCs (nonsolid access surface) used 241 doses. As a result, the tPA utilisation rate was significantly lower in hospitals using MaxPlus™/MaxZero™ NCs versus other NCs (1.99 versus 4.63 doses per 100 patient-days; P = 0.014). As shown in Figure 4A, the tPA utilisation rate in hospitals using solid-access-surface NCs was only 30%.
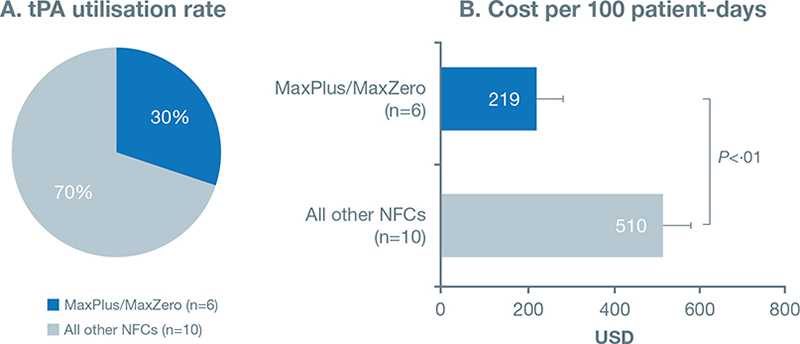
Fig. 4. tPA utilisation rates were significantly lower in hospitals using the MaxPlus/MaxZero NFC. A. tPA utilisation rate for the 16 hospitals surveyed. B. Cost per 100 patient-days for MaxPlus/MaxZero vs other NFCs.
Further, cost based on hospital tPA use was analyzed. The average cost (per 100 patient-days) with MaxPlus™/MaxZero™ NCs was $219, whereas that with other NCs was significantly higher, $510 (P = 0.01) (Table 4). This resulted in lower annual cost for tPA in hospitals using MaxPlus™/MaxZero™ NCs.
Discussion
Cross-sectional studies such as the current descriptive report are valuable, as they allow cost-efficient comparison of many variables in a large subject pool. The current study used a survey to compare the impact of various NC types on catheter-related CLABSI and occlusion. However, this study was not without limitations. Because the survey was descriptive and variables not controlled for or manipulated, it was difficult to determine whether NC design (e.g. solid access surface) was the only variable responsible for CVC-IBO and CLABSI rates. Survey data were collected regionally in California, so a broader survey would better represent different populations. Pre-existing conditions, including pulmonary disease (25), parenteral nutrition, (26) or multiple concurrent CVCs (27), can increase CLABSI risk. In the future, data on patient characteristics and NC training practices should be collected from each hospital. Lastly, the cost analysis in the current study includes the cost associated with only tPA use.
CVCs are an essential component of hospital patient care; catheter occlusion and CLABSI are typical complications associated with CVC use. NCs widely used with CVCs were originally marketed to reduce the risk of needlestick injury and exposure to bloodborne pathogens among healthcare workers (7, 8). However, NCs used in clinical practice, although designed to improve clinical outcomes, have been associated with CLABSI outbreak and catheter occlusion in acute-care hospitals (7, 8, 14, 28). Subsequently, various NC design changes were implemented to reduce infection and occlusion. Certain design features of the new-generation NCs are a visible fluid pathway: a solid, flat, smooth access surface; one-part activation of the fluid pathway; and an open fluid pathway (11). The current study investigated the incidence of CVC-IBO and CLABSI associated with five NCs of varying design. Sixteen hospitals of different capacities across Northern and Southern California were surveyed. Each hospital used one of the five NC varieties listed: CARESITE, Clave/MicroClave, MaxPlus™/MaxZero™, InVision-Plus Clear, or ULTRASITE.
The five NCs used by the surveyed hospitals vary in external and internal design, which might be responsible for the different CLABSI and occlusion rates reported here. NCs with simple designs and few internal components are easier to clean and flush and might be associated with lower CLABSI and occlusion rates. In contrast, NCs with complex internal designs, such as mechanical valves, rely on multiple moving parts to regulate fluid flow and have larger dead space that could harbor bacteria (24, 29). In the current study, the NCs with the lowest CLABSI and occlusion rates were the BD MaxPlus™/MaxZero™ devices. These connectors, unlike the other NCs discussed in this report, are designed with a solid, flat, smooth external access surface that can be efficiently cleaned, a dual-seal bounce-back design to reduce bacterial ingress, and a clear housing that enables observation of flushing. Similar to other connectors discussed in this article, MaxPlus™/MaxZero™ NCs also create positive displacement. Our hypothesis is that the solid, flat, smooth external surface with the dual-seal design unique to the MaxPlus™/MaxZero™ NCs, along with the clear housing and simple internal design, enabled complete flushing and prevented bacterial ingress, which resulted in lower CLABSI and occlusion rates.
New-generation NCs are specifically designed to improve clinical outcomes and reduce CLABSI risk. The current study surveyed 16 hospitals and determined that their mean CLABSI rate was 2.34 per 1,000 CLDs, which was higher than the national average of 0.8 per 1,000 CLDs (19). The cause of the higher CLABSI rate reported by the hospitals in this study could not be clearly elucidated. It is interesting that the hospitals that used the MaxPlus™/MaxZero™ devices, the only NCs in this survey with a solid access surface, had significantly lower CLABSI rates versus those with a nonsolid access surface (CLABSI rate for MaxPlus™/MaxZero™ versus others: 1.32 versus 2.95, respectively, Fig. 3). Even in other studies, the MaxPlus™ device has been associated with significantly lower CLABSI rates (11). Findings of previous evidence-based reports have suggested that near-zero CLABSI rates could be achieved by using positive-displacement connectors, along with additional interventions (30). Although MaxPlus™/MaxZero™ are positive-displacement NCs, in the current study, there were no significant differences in CLABSI rates associated with NC displacement type. Unlike data in previous studies that have associated negative- or positive-displacement NCs with increased infection (10), the current study data strongly suggest that external NC design (access surface) can influence CLABSI rate.
An array of clinical outcomes of catheter occlusion associated with NCs has been published. Studies have reported a lower occlusion rate with mechanical-valve NCs versus split-septum NCs, a lower occlusion rate with positive-displacement NCs versus negative-displacement NCs, and no difference in occlusion rates between positive- and negative- or neutral-displacement NCs (31–33). In the current study, the occlusion rate in hospitals using the BD MaxPlus™/MaxZero™ device was 1.51 per 1,000 CLDs, whereas the rate in hospitals using other nonsolid access NCs was significantly higher, at 4.04 per 1,000 CLDs. Our results agree with those of a previous study that found an association of reduced occlusion rate with the use of solid-surface NCs over split-septum NCs (24).
Reducing rates of CLABSI and catheter-related occlusion can result in cost saving. CLABSI incidence increases the duration of hospital stay and per a meta-analysis report can cost approximately $48,108 (95% CI: $27,232 to $68,983). Hospital-acquired CLABSI also increases excess mortality associated with CLABSI to 0.5 (150 excess deaths for every 1,000 CLABSI cases) (34). Catheter occlusion is a common complication of CVCs and can occur within 1 to 2 years of placement in 14 – 36% of patients with long-term CVCs (35). An occluded catheter can compromise patient care, disrupt medication administration, and lead to infection. Dissolving catheter occlusion requires the use of tPAs such as Alteplase and can be expensive: One 100-mg vial of tPA can cost around $7,000 (36). According to survey findings, the tPA utilisation rate with MaxPlus™/MaxZero™ was 1.99 doses per 100 patient days versus 4.63 doses per 100 patient days with other NCs. (P = 0.047). Thus MaxPlus™/MaxZero™ NCs can reduce the need for tPA by approximately 60%, which would ultimately result in cost saving (Fig. 4B). Along with safer NCs, other factors, such as continuous training of healthcare workers, implementing good hand hygiene, and adequate cleaning and disinfection protocol, may be easy, cost-effective methods of curbing device-related infection (5, 37).
Conclusion
In conclusion, findings of the current study suggest that solid-surface NCs have better clinical outcomes as compared with nonsolid-surface NCs, irrespective of displacement type. In the future, well-designed randomized controlled clinical studies will be needed to further evaluate the impact of various NCs on CLABSI and occlusion.
Ethical approval
The study did not require IRB approval due to the nature of the study only utilizing extracted data without identification of patient-specific identifying information.
References
| 1. | Centers for Disease Control and Prevention. Workbook for designing, implementing, and evaluating a sharps injury prevention program. Available from: https://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf [cited 4 May 2021]. |
| 2. | OSHA. Healthcare wide hazards needlestick/sharps injuries CDC 2008. Available from: https://www.osha.gov/SLTC/etools/hospital/hazards/sharps/sharps.html [cited 4 May 2021]. |
| 3. | Siegel GS, Leinsing KR, inventors; CareFusion 303 Inc., current assignee. Needleless connector. US Patent US5549577A. California: San Diego, December 29, 1993. |
| 4. | Needlestick Safety and Prevention Act, H.R. 5178, 106th Congress. (2000). Available from: https://www.congress.gov/bill/106th-congress/house-bill/5178 [cited 26 June 2021]. |
| 5. | Kelly LJ, Jones T, Kirkham S. Needle-free devices: keeping the system closed. Br J Nurs 2017; 26: S14–9. doi: 10.12968/bjon.2017.26.2.S14 |
| 6. | Tabak YP, Jarvis WR, Sun X, Crosby CT, Johannes RS. Meta-analysis on central line−associated bloodstream infections associated with a needleless intravenous connector with a new engineering design. Am J Infect Control 2014; 42: 1278–84. doi: 10.1016/j.ajic.2014.08.018 |
| 7. | Jarvis WR. Needleless connectors and the improvement of patient and healthcare professional safety. Infect Control Today 2013;17: 1–3. |
| 8. | Hadaway L, Richardson D. Needleless connectors: a primer on terminology. J Infus Nurs 2010; 33: 22–31. doi: 10.1097/NAN.0b013e3181c65cc9 |
| 9. | Elli S, Abbruzzese C, Cannizzo L, Lucchini A. In vitro evaluation of fluid reflux after flushing different types of needleless connectors. J Vasc Access 2016; 17: 429–34. doi: 10.5301/jva.5000583 |
| 10. | Jarvis WR, Murphy C, Hall KK, Fogle PJ, Karchmer TB, Harrington G. Health care-associated bloodstream infections associated with negative- or positive-pressure or displacement mechanical valve needleless connectors. Clin Infect Dis 2009; 49: 1821–7. doi: 10.1086/648418 |
| 11. | Rosenthal VD. Clinical impact of needle-free connector design: a systematic review of literature. J Vasc Access 2020; 21: 847–53. doi: 10.1177/1129729820904904 |
| 12. | Casey AL, Karpanen TJ, Nightingale P, Chaganti S, Elliott TSJ. Microbiologic contamination of a positive- and a neutral-displacement needleless intravenous access device in clinical use. Am J Infect Control 2016; 44: 1678–80. doi: 10.1016/j.ajic.2016.06.027 |
| 13. | Casey AL, Karpanen TJ, Nightingale P, Elliott TSJ. The risk of microbial contamination associated with six different needle-free connectors. Br J Nurs 2018; 27: S18–26. doi: 10.12968/bjon.2018.27.2.S18 |
| 14. | Btaiche IF, Kovacevich DS, Khalidi N, Papke LF. The effects of needleless connectors on catheter-related thrombotic occlusions. J Infus Nurs 2011; 34: 89–96. doi: 10.1097/NAN.0b013e31820b3ea9 |
| 15. | Khalidi N, Kovacevich DS, Papke-O’Donnell LF, Btaiche IF. Impact of the positive pressure valve on vascular access device occlusions and bloodstream infections. J Vasc Access 2009; 14: 84–91. doi: 10.2309/java.14-2-6 |
| 16. | Holt D, Lawrence S. The influence of a novel needleless valve on central venous catheter occlusions in pediatric patients. J Vasc Access 2015; 20: 214–20.e2. doi: 10.1016/j.java.2015.07.003 |
| 17. | Deitcher SD, Fesen MR, Kiproff PM, Hill PA, Li X, McCluskey ER. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol 2002; 20: 317–24. doi: 10.1200/JCO.2002.20.1.317 |
| 18. | Plohal A, Schiller K. Efficacy of reducing alteplase dose to restore patency in nonhemodialysis central vascular access devices. J Infus Nurs 2017; 40: 112–5. doi: 10.1097/NAN.0000000000000209 |
| 19. | Haddadin Y, Annamaraju P, Regunath H. Central line associated blood stream infections. StatPearls website. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430891/ [cited 4 January 2021]. |
| 20. | Madni T, Eastman AL. CLABSI. In: Salim A, Brown C, Inaba K, Martin M, editors. Surgical critical care therapy. 1st ed. Springer International Publishing AG; Germany: Heidelberg, 2018; p. 399–402. |
| 21. | Rosenthal VD, Al-Abdely HM, El-Kholy AA, AlKhawaja SAA, Leblebicioglu H, Mehta Y. International nosocomial infection control consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am J Infect Control 2016; 44: 1495–504. doi: 10.1016/j.ajic.2016.08.007 |
| 22. | Centers for Disease Control and Prevention. Central line-associated bloodstream infections. Centers for Disease Control and Prevention website. Available from: https://arpsp.cdc.gov/profile/infections/CLABSI?print=true [cited 7 December 2020]. |
| 23. | Baskin JL, Pui C-H, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009; 374: 159–69. doi: 10.1016/S0140-6736(09)60220-8 |
| 24. | Williams A. Catheter occlusion in home infusion: the influence of needleless connector design on central catheter occlusion. J Infus Nurs 2018; 41: 52–7. doi: 10.1097/NAN.0000000000000259 |
| 25. | Baier C, Linke L, Eder M, Schwab F, Chaberny IF, Vonberg R-P. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS One 2020; 15: e02277772. doi: 10.1371/journal.pone.0227772 |
| 26. | Fonseca G, Burgermaster M, Larson E, Seres DS. The relationship between parenteral nutrition and central line-associated bloodstream infections: 2009–2014. JPEN J Parenter Enteral Nutr 2018; 42: 171–5. doi: 10.1177/0148607116688437 |
| 27. | Concannon C, van Wijngaarden E, Stevens V, Dumyati G. The effect of multiple concurrent central venous catheters on central line-associated bloodstream infections. Infect Control Hosp Epidemiol 2014; 35: 1140–6. doi: 10.1086/677634 |
| 28. | Salgado CD, Chinnes L, Paczesny TH, Cantey JR. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve device at a long-term acute care hospital. Infect Control Hosp Epidemiol 2007; 28: 684–8. doi: 10.1086/516800 |
| 29. | Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs 2021; 44: S1–224. doi: 10.1097/NAN.0000000000000396 |
| 30. | Alanazi TNM, Alharbi KAS, Alrawaili ABR, Arishi AAM. Preventive strategies for the reduction of central line-associated bloodstream infections in adult intensive care units: a systematic review. Collegian 2020; 28: 438–446. doi: 10.1016/j.colegn.2020.12.001 |
| 31. | Jacobs BR, Schilling S, Doellman D, Hutchinson N, Rickey M, Nelson S. Central venous catheter occlusion: a prospective, controlled trial examining the impact of a positive-pressure valve device. JPEN J Parenter Enteral Nutr 2004; 28: 113–8. doi: 10.1177/0148607104028002113 |
| 32. | Schilling S, Doelleman D, Hutchinson N, Jacobs BR. The impact of needleless connector device design on central venous catheter occlusion in children: a prospective, controlled trial. J Parenter Enteral Nutr 2006; 30: 85–90. doi: 10.1177/014860710603000285 |
| 33. | Buehrle DC. A prospective, randomized comparison of three needleless IV systems used in conjunction with peripherally inserted central catheters. J Assoc Vasc Access 2004; 9: 35–8. doi: 10.1177/014860710603000285 |
| 34. | Agency for Healthcare Research and Quality. Estimating the additional hospital inpatient cost and mortality associated with selected hospital-acquired conditions. Available from: https://www.ahrq.gov/hai/pfp/haccost2017-results.html [cited 11 June 2021]. |
| 35. | Ernst FR, Chen E, Lipkin C, Tayama D, Amin AN. Comparison of hospital length of stay, costs, and readmissions of alteplase versus catheter replacement among patients with occluded central venous catheters. J Hosp Med 2014; 9: 490–6. doi: 10.1002/jhm.2208 |
| 36. | Leslie-Mazwi TM, Chandra RV, Hirsch JA. To tPA or not to tPA, that is the question. Am J Neuroradiol 2017; 38: 1464–6. doi: 10.3174/ajnr.A5263 |
| 37. | Mohapatra S, Kapil A, Suri A, Pandia MP, Bhatia R, Borkar S. Impact of continuous education and training in reduction of central line-associated bloodstream infection in neurointensive care unit. Indian J Crit Care Med 2020; 24: 414–7. doi: 10.5005/jp-journals-10071-23455 |