ORIGINAL ARTICLE
Performance of the manual and Sofia rapid antigen test in medical staff exposed to Omicron variants
Humberto Guanche Garcell1* , Eduardo Vargas Girón1
, Eduardo Vargas Girón1 , Sandra Ibis Gonzalez Perez1
, Sandra Ibis Gonzalez Perez1 , Angel M Felipe Garmendia1
, Angel M Felipe Garmendia1 and Francisco Gutierrez García2
and Francisco Gutierrez García2
1Infection Control Department, The Cuban Hospital, Qatar; 2National Institute of Nephrology, La Habana, Cuba
Abstract
Background: Variable COVID-19 rapid antigen test sensitivity had been reported and the effect of viral variants drew attention to the impact in the early detection of cases.
Objective: The study aims to compare the performance of antigen tests (manual rapid antigen tests [RAT], and Sofia test) in medical staff exposed during the circulation periods of different Omicron variants.
Methods: The descriptive study of samples collected for the diagnosis of SARS-CoV-2 infection included medical staff at The Cuban Hospital (TCH) Hospital repeated from December 2021 to December 2022, including cases confirmed by SARS CoV-2 polymerase chain reaction (PCR), and a RAT. December 2021–March 2022 and June-December 2022 were considered the periods of Omicron BA.1.1. variants and Omicron BA.4/5 respectively. Comparison of Ct figures between categories was carried out using the Wilcoxon–Mann–Whitney test, and sensitivity (95% confidence intervals [CI]) values were calculated. Results: 287 healthcare workers were diagnosed with COVID-19 during the study period, 56.1% during the B.1.1. variant period, and 43.9% when B.4/5 variants were predominantly in circulation. Sensitivity of the manual RAT test (82.5%; 95% CI 73.4–89.4) was higher during the B.1.1. variant circulation in comparison with the B.4/5 period (68.9%;53.4–81.8). These two methods during this B.4/5 period had quite similar sensitivity figures when compared to each other; manual 68.9% (95% CI 53.4–81.8) and Sofia 72.7% (95% CI 60.4–83.0).
Conclusion: The variation in sensitivity of the RAT for SARS CoV-2 variants and the similar performance of manual and SOFIA methods of RAT could be considered in the diagnostic approach of COVID-19 and the appropriate isolation of potentially infectious cases.
Keywords: rapid antigen test; Sofia; real-time PCR; SARS CoV-2; COVID-19; medical staff; Qatar
Citation: Int J Infect Control 2024, 20: 23502 – http://dx.doi.org/10.3396/ijic.v20.23502
Copyright: © 2024 Humberto Guanche Garcell et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 6 April 2023; Accepted: 6 May 2024; Published: 27 November 2024
Competing interests and funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study. The authors report no conflict of interests.
*Humberto Guanche Garcell, The Cuban Hospital, PO Box 27555, Dukhan, Qatar. Email: humbertoguanchegarcell@yahoo.es; guanche@infomed.sld.cu
Early detection and prompt isolation constituted key components for preventing infectious disease transmission. During COVID-19, various challenges were faced including the development of diagnostic technology for a new disease and the effect on test accuracy of viral variants (1, 2). The standard for SARS CoV-2 diagnosis is a reverse transcriptase polymerase chain reaction (PCR) assay performed with nasopharyngeal swabs. Other technologies include antigen and immunoassay-based and antibody-based detection (1, 2).
The use of rapid antigen tests (RAT) and point-of-care (POC) diagnostic tests was recommended by the World Health Organization (WHO) to face the limitations of trained laboratory staff in performing molecular tests during the pandemic and to improve the turnaround times (3). Various reports describe variable antigen test sensitivity according to various factors, especially the viral load (4–6). Moreover, according to published reports the antigen test accuracy in real life is lower than the manufactured data (5, 6)
A false-negative test impacts infection prevention and control in healthcare facilities generating a significant risk of transmission to patients, visitors, and staff members. The WHO recommends RAT that meets minimum performance requirements of ≥ 80% sensitivity and ≥ 97% specificity (7, 8).
In healthcare settings, the early diagnosis of staff exposed to COVID-19 plays a remarkable role in preventing transmission to patients, visitors, and staff. The impact of COVID-19 on health workers was significant worldwide, and in the State of Qatar, where 10.6% of staff from Hamad Medical Corporation (HMC) (the main healthcare provider in the country) had been confirmed to be COVID positive (9, 10).
During 2022, two peaks of COVID-19 had been documented in the medical staff at The Cuban Hospital (TCH) (an HMC member); this was during a period of predominant circulation of Omicron variants (B.1.1.529, BA.1, BA.1.1 and BA.4/5 lineages). In addition to the SARS CoV-2 PCR test, during the first peak (January–March 2022) antigen test (RAT) was used for diagnosing, and during the second peak (June–December 2022) Sofia SARS rapid antigen fluorescent immunoassay (FIA) test was introduced (7). A study was conducted to compare the performance of antigen tests (manual RAT, and Sofia test) in medical staff exposed during the circulation periods of different Omicron variants.
Methods
This is a descriptive study of samples collected for the diagnosis of SARS-CoV-2 infection in medical staff at TCH from December 2021 to December 2022. It involves cases confirmed by SARS CoV-2 PCR, and a RAT, either by manual method or SOFIA. December 2021 to March 2022 was considered the period of circulation of Omicron BA.1.1. variants and the June to December 2022 period was considered the period of circulation of Omicron BA.4/5 variants circulation.
The staff category (nurse, physician, technologist), confirmation test (RAT or PCR SARS CoV-2 test), probable source (hospital-acquired, community-acquired), infection type either primo infection (COVID-19 infection without laboratory evidence of previous infection) or reinfection (new COVID-19 infection with previous laboratory-confirmed infection) were extracted from the infection control department records. Data of demographics, test results at diagnosis, and follow-up RAT (usually performed 7–10 days after COVID-19 diagnosis), including the PCR cycle threshold value (Ct) with a positivity cutoff of less than 30, and COVID-19 vaccine received were collected from the staff electronic medical records. COVID-19 infection was defined as pre-vaccination when the staff received neither the COVID-19 vaccine nor completed the primary vaccination; however, post-vaccination was determined when the staff received the primary vaccination before COVID-19 confirmation.
The staff were trained to carry out the RAT in situ by the manual method (Panbio™ COVID-19 Ag Rapid Test Device) or by using the Sofia antigen FIA (Quidel Corporation) (11, 12). The Sofia FIA is a sandwich-based lateral flow assay and provides automated and user-independent read-out using the Sofia 2 FIA analyzer.
The study was approved by the Medical Research Center (Hamad Medical Corporation, Doha, Qatar) (MRC-01-22-593).
Analysis: Descriptive statistical methods for data analysis were used in the statistical packages IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA), and MedCal version 12.1.0.0. (https://www.medcalc.org). Comparison of Ct figures between categories was carried out using the Wilcoxon–Mann–Whitney test, and sensitivity values at 95% confidence intervals (CI) were calculated.
Results
A total of 287 healthcare workers were diagnosed with COVID-19 during the study period, of which 167 were nurses (58.2%), 62 (21.6%) physicians, and 58 (20.2%) technologists. The mean age was 46.3 years (standard deviation [SD] 6.2 years), without differences among categories. Female sex predominated (68.6%) mainly among nurses and technologists (Table 1). COVID-19 infection was confirmed by PCR in 239 (83.3%) workers, out of which 199 (83.2%) had Ct figures ≤30 and 28 (11.7%) had Ct figures >30. Among the ones with lower Ct figures, a higher RAT positivity was observed, and vice versa (Figure 1). A follow-up RAT test was performed in a mean time of 6.5 (1.3) days in 254 (88.5%) staff members and 97 (38.2%) had a positive test.
| Variables* | Nurse | Physician | Technologist | Total |
| n = 167 | n = 62 | n = 58 | n = 287 | |
| Age (mean ± SD) (years) | 46.2 (5.0) | 48.2 (7.8) | 44.4 (6.7) | 46.3 (6.2) |
| Female sex | 126 (75.4) | 33 (53.2) | 38 (65.5) | 197 (68.6) |
| Male sex | 41 (24.6) | 29 (46.8) | 20 (34.5) | 90 (31.4) |
| Probable source of COVID-19 infection | ||||
| Community | 57 (34.1) | 30 (48.4) | 33 (56.9) | 120 (41.8) |
| Hospital | 104 (62.3) | 32 (51.6) | 22 (37.9) | 158 (55.1) |
| Unknown | 6 (3.6) | 0 (0.0) | 3 (5.2) | 9 (3.1) |
| Infection | ||||
| Primo infection | 146 (87.4) | 58 (93.5) | 51 (87.9) | 255 (88.9) |
| Reinfection | 21 (12.6) | 4 (6.5) | 7 (12.1) | 32 (11.1) |
| Disease | ||||
| Pre-vaccination | 3 (1.8) | 1 (1.6) | 0 (0.0) | 4 (1.4) |
| Postvaccination | 164 (98.2) | 61 (98.4) | 58 (100) | 283 (98.6) |
| Vaccine received | ||||
| Pfizer | 160 (95.8) | 60 (96.8) | 54 (93.1) | 274 (95.5) |
| Moderna | 7 (4.2) | 0 (0.0) | 3 (5.2) | 10 (3.5) |
| Abdalla | 0 (0.0) | 2 (3.2) | 0 (0.0) | 2 (0.7) |
| Aztra Seneca | 0 (0.0) | 0 (0.0) | 1 (1.7) | 1 (0.3) |
| Probable COVID-19 variants | ||||
| B.1.1. | 94 (56.3) | 33 (53.2) | 34 (58.6) | 161 (56.1) |
| B.4/5. | 73 (43.7) | 29 (46.8) | 24 (41.4) | 126 (43.9) |
| *data presented as number (%) unless specified. | ||||
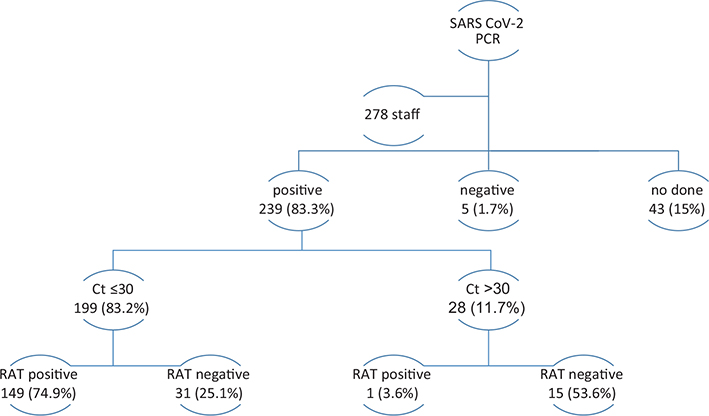
Fig. 1. Diagnostic test in the COVID-19 staff.
Hospital-acquired exposure (during direct patient contact, contact with health workers in the hospital) was identified in 55.1%, and community exposure in 41.8%, either in common places of the community (e.g. markets) or in shared staff accommodation. Hospital exposure was more frequent among physicians and nurses, while the community predominates among technologists. Out of 32 staff members (11.1%) with confirmed COVID-19 reinfection, 95.5% received the Pfizer BionTech vaccine. The diagnosis of COVID-19 was done during the period of predominant circulation of the B.1.1. variant in 56.1% of staff, and 43.9% during B.4/5 variants predominant circulation (Table 1).
The time between a more recent vaccine dose and COVID-19 diagnosis was 192.8 days (SD 123.8 days) for Pfizer vaccinated staff and 193.0 days (SD 86.8 days) (p = 0.73) for Moderna vaccinated staff.
Ct figures were lower in staff with positive RAT in manual and Sofia test on diagnosis when compared with those with negative RAT tests (p = 0.00) (Figure 2A and B). Also, Ct figures were lower in staff with primo-infection (Ct = 22.6 (SD 5.5) in comparison with reinfection (25.2 (6.3) (p = 0.03) (Figure 2C). The Ct figures in post-vaccinated staff confirmed during B.1.1 variants (23.2 (5.4) and B.4/5 variants (22.4 (5.8) were similar (p = 0.18) (Figure 2D). Ct figures analysis of pre-vaccinated cases was not feasible due to the low number of cases.
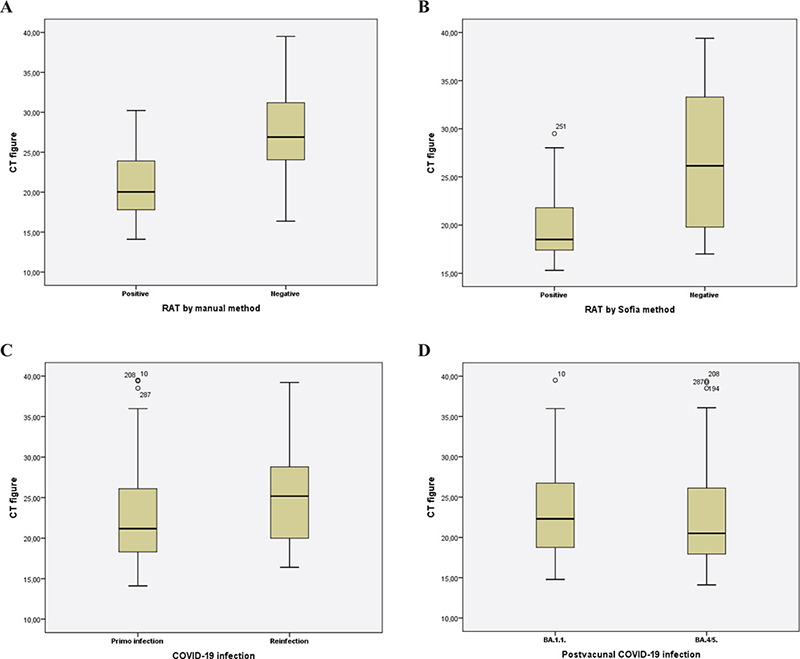
Fig. 2. Box plot to describe the PCR cycle threshold figures according to antigen test methods (A, B), COVID-19 infection type (C), and potential infectious variant (D).
Figure 3 shows the sensitivity, and 95% CI, for the antigen test performed, according to the method used for antigen detection, and the period of COVID-19 variants circulation. It can be noted that the sensitivity of the manual RAT test (82.5%; 95% CI 73.4–89.4) was higher during B.1.1. variant circulation in comparison with the B.4/5 period (68.9%;53.4–81.8). These two methods during this B.4/5 period had quite similar sensitivity figures when compared to each other; manual 68.9% (95% CI 53.4–81.8) and Sofia 72.7% (95% CI 60.4–83.0).
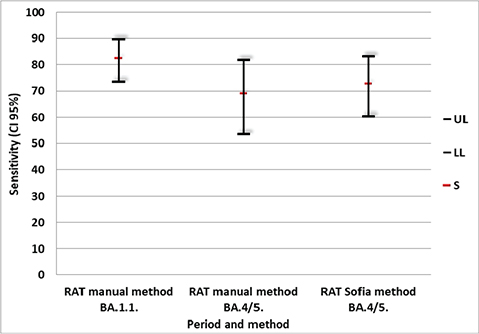
Fig. 3. Sensitivity (95% confidence interval) for SARS-CoV-2 rapid antigen test according to the method and COVID-19 variants circulation periods. S, sensitivity; LL, Lower limit; UL, Upper limit.
Discussion
Our study describes higher sensitivity of RAT performed by manual method during periods of Omicron B.1.1. variant circulation compared with B.4/5 variant circulation, and no differences in sensitivity among manual or Sofia method of RAT during B.4/5 circulation.
The sensitivity of RAT in real life reaches variable values ranging from 30 to 80% according to published papers, with figures from manufacturers being much higher (over 90%) (7, 13, 14). Various studies confirm the variable sensitivity of PCR and antigen test in symptomatic patients in comparison with asymptomatic patients, and patients with Ct figures under 30 in comparison with patients over 30 (15–18). Studies conducted using Panbio™ COVID-19 Ag Rapid Test Device have described variable results (19, 20). Albert et al. in primary care services report a sensitivity of 79.6% (95%CI 67.0–88.8%) during the period of Non-Omicron variants circulation, while Galliez RM describes 89% of sensitivity for nasal tests in symptomatic patients during Omicron circulation (19, 20). Most of the COVID-19 staff studied had symptoms, which indicated high sensitivity in our study in comparison with asymptomatic patients. Similar findings have been reported for Sofia RAT with 72.1% sensitivity in symptomatic patients according to Brihn A et al., and 57.1% in emergency department patients reported by Bornemann et al. (6, 7).
New COVID-19 variants have generated great concern due to their potential impact on the performance reduction of diagnostic tests, especially for rapid diagnostic tests. Differences in sensitivity had been observed for Omicron compared with Alpha and Delta variants (21–23). Leuzinger et al. showed lower antigen detection rates for Omicron BA.2 and BA.5 in samples with Ct figures <29, probably related to the variation within the nucleocapsid protein (24). This research confirms the variable sensitivity of RAT in Omicron variants during different circulation periods in healthcare workers, but similar performance of Panbio™ COVID-19 Ag Rapid Test Device and Sofia test. The reduction in the sensitivity of diagnostic tests exposed to new variants of SARS CoV-2 may affect their clinical value in the timely detection and isolation of patients and the prevention of transmission in healthcare and community setting.
This study has few limitations to consider. First, it is a single-center study including healthcare workers and not the general population which limits the comparison; nevertheless, the study includes all healthcare workers confirmed during the study period providing valuable data on the matter. Second, the exclusion of other clinical variables limits the likely analysis of Ct figures with special reference to asymptomatic or symptomatic COVID-19 cases.
Conclusion
The variation in sensitivity of the RAT for SARS CoV-2 variants and the similar performance of manual and SOFIA methods of RAT could be considered in the diagnostic approach of COVID-19 and the appropriate isolation of potentially infectious cases.
Acknowledgements
The authors would like to thank Alexis Gonzalez Velázquez for the proofreading of the manuscript.
Authors’ Contributions
Study design: HGG. Data acquisition: HGG, EVG, SIGP. Data analysis: HGG, FGG. Manuscript writing: HGG, FGG. Critical review and major scientific input: EVG, SIGP, AMFG, FGG.
References
| 1. | Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med 2022; 54(1): 524–40. doi: 10.1080/07853890.2022.2031274 |
| 2. | Rai P, Kumar BK, Deekshit VK, Karunasagar I, Karunasagar I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl Microbiol Biotechnol 2021; 105(2): 441–55. doi: 10.1007/s00253-020-11061-5 |
| 3. | Smith RD, Johnson JK, Clay C, Girio-Herrera L, Stevens D, Abraham M, Zimand P, et al. Clinical evaluation of Sofia Rapid Antigen Assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among emergency department to hospital admissions. Infect Control Hosp Epidemiol 2022; 43(8): 968–73. doi: 10.1017/ice.2021.281 |
| 4. | Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021; 3(3): CD013705. doi: 10.1002/14651858.CD013705.pub2. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. |
| 5. | Jegerlehner S, Suter-Riniker F, Jent P, Bittel P, Nagler M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis 2021; 109: 118–22. doi: 10.1016/j.ijid.2021.07.010 |
| 6. | Bornemann L, Kaup O, Kleideiter J, Panning M, Ruprecht B, Wehmeier M. Real-life evaluation of the Sofia SARS-CoV-2 antigen assay in a large tertiary care hospital. J Clin Virol 2021; 140: 104854. doi: 10.1016/j.jcv.2021.104854 |
| 7. | Brihn A, Chang J, OYong K, Balter S, Terashita D, Rubin Z, et al. Diagnostic performance of an antigen test with RT-PCR for the detection of SARS-CoV-2 in a hospital setting – Los Angeles County, California, June-August 2020. MMWR Morb Mortal Wkly Rep 2021; 70(19): 702–6. doi: 10.15585/mmwr.mm7019a3 |
| 8. | World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. Interim guidance 6 October 2021. Available from: https://iris.who.int/bitstream/handle/10665/345948/WHO-2019-nCoV-Antigen-Detection-2021.1-eng.pdf [cited 15 January 2024]. |
| 9. | Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021; 190(1): 161–75. doi: 10.1093/aje/kwaa191 |
| 10. | Alajmi J, Jeremijenko AM, Abraham JC, Alishaq M, Concepcion EG, Butt AA, et al. COVID-19 infection among healthcare workers in a national healthcare system: the Qatar experience. Int J Infect Dis 2020; 100: 386–9. doi: 10.1016/j.ijid.2020.09.027 |
| 11. | Panbio. Panbio COVID-19 Ag (nasal) instructions for use. 41FK11/41FK21-01-EN-A1.2021.01. Jena: Abbott Rapid Diagnostics Jena GmbH; 2021. |
| 12. | Sofia®. SARS Antigen FIA [package insert, EUA]. San Diego, CA: Quidel Corporation; 2020. |
| 13. | Fourati S, Langendorf C, Audureau E, Challine D, Michel J, Soulier A, et al. Performance of six rapid diagnostic tests for SARS-CoV-2 antigen detection and implications for practical use. J Clin Virol 2021; 142: 104930. doi: 10.1016/j.jcv.2021.104930 |
| 14. | Landaas ET, Storm ML, Tollånes MC, Barlinn R, Kran AB, Bragstad K, et al. Diagnostic performance of a SARS-CoV-2 rapid antigen test in a large, Norwegian cohort. J Clin Virol 2021; 137: 104789. doi: 10.1016/j.jcv.2021.104789 |
| 15. | Möckel M, Corman VM, Stegemann MS, Hofmann J, Stein A, Jones TC, et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers 2021; 26(3): 213–20. doi: 10.1080/1354750X.2021.1876769 |
| 16. | Krüttgen A, Cornelissen CG, Dreher M, Hornef MW, Imöhl M, Kleines M. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J Virol Methods. 2021; 288: 114024. doi: 10.1016/j.jviromet.2020.114024 |
| 17. | Wölfl-Duchek M, Bergmann F, Jorda A, Weber M, Müller M, Seitz T, et al. Sensitivity and specificity of SARS-CoV-2 rapid antigen detection tests using oral, anterior nasal, and nasopharyngeal swabs: a Diagnostic Accuracy Study. Microbiol Spectr 2022; 10(1): e0202921. doi: 10.1128/spectrum.02029-21 |
| 18. | Hartard C, Berger S, Josse T, Schvoerer E, Jeulin H. Performance evaluation of an automated SARS-CoV-2 Ag test for the diagnosis of COVID-19 infection on nasopharyngeal swabs. Clin Chem Lab Med 2021; 59(12): 2003–9. doi: 10.1515/cclm-2021-0569 |
| 19. | Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect 2021; 27(3): 472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004 |
| 20. | Galliez RM, Bomfim L, Mariani D, Leitão IC, Castiñeiras ACP, Gonçalves CCA, Ortiz da Silva B, et al. Evaluation of the Panbio COVID-19 antigen rapid diagnostic test in subjects infected with omicron using different specimens. Microbiol Spectr 2022; 10(3): e0125022. doi: 10.1128/spectrum.01250-22 |
| 21. | Cocherie T, Bastide M, Sakhi S, Zafilaza K, Flandre P, Leducq V, et al. Decreased sensitivity of rapid antigen test is associated with a lower viral load of omicron than delta SARS-CoV-2 variant. Microbiol Spectr 2022; 10(5): e0192222. doi: 10.1128/spectrum.01922-22 |
| 22. | Soni A, Herbert C, Filippaios A, Broach J, Colubri A, Fahey N, et al. Comparison of Rapid antigen tests’ performance between delta and omicron variants of SARS-CoV-2: a secondary analysis from a serial home self-testing study. Ann Intern Med 2022; 175(12): 1685–92. doi: 10.7326/M22-0760 |
| 23. | Kyritsi MA, Speletas M, Mouchtouri V, Vachtsioli E, Babalis D, Kouliou O, et al. Performance evaluation of a rapid antigen test (RAT) during omicron pandemic wave in Greece, conducted by different personnel, and comparison with performance in previous wave (Alpha Variant) period. Diagnostics (Basel) 2022; 12(5): 1048. doi: 10.3390/diagnostics12051048 |
| 24. | Leuzinger K, Roloff T, Egli A, Hirsch HH. Impact of SARS-CoV-2 omicron on rapid antigen testing developed for early-pandemic SARS-CoV-2 variants. Microbiol Spectr 2022; 10(4): e0200622. doi: 10.1128/spectrum.02006-22 |