ORIGINAL ARTICLE
Mild respiratory illness in SARS-CoV-2 infection after vaccination in healthcare workers
Humberto Guanche Garcell1* and Reynaldo Barban Arias2
1Hospital Docente Clínico Quirúrgico Joaquín Albarrán, La Habana, Cuba; 2Hospital Giraldo Aponte Fonseca, Santiago de Cuba
Abstract
COVID-19 after vaccination is a consequence of multiple factors, including the variable vaccine efficacy and the emergence of new viral variants. Sixteen cases of infection after completing the primary series of vaccination in healthcare workers (HCWs) are described. Ten cases had symptoms, mainly loss of smell (four cases), cough (four cases), fever (two cases), nasal discharge or obstruction (three cases), general malaise (two cases), and dyspnea and loss of taste in one case each. The median time between the second dose of the primary vaccination and the positive severe acute respiratory syndrome coronavirus 2 polymerase-chain reaction (PCR) was 132.5 days, and the median cycle threshold value at the time of diagnosis was 25.1. Laboratory tests performed at diagnosis showed results mostly in normal parameters, and in 10 cases, pulmonary findings suggestive of COVID-19 were described. The clinical course of the disease was satisfactory, without complications or sequelae at discharge.
Conclusion: COVID-19 after vaccination in HCWs was mild, with a favorable course of the disease.
Keywords: COVID-19; infection; vaccination; healthcare workers; Qatar
Citation: Int J Infect Control 2022, 18: 22256 – http://dx.doi.org/10.3396/ijic.v18.22256
Copyright: © 2022 Humberto Guanche Garcell and Reynaldo Barban Arias. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 26 November 2021; Revised: 12 May 2022; Accepted: 23 July 2022; Published: 19 October 2022
Competing interests and funding: None to declare for all authors.
*Humberto Guanche Garcell Email: guanche@infomed.sld.cu; humbertoguanchegarcell@yahoo.es
The vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitutes a fundamental resource to control the current pandemic, and various vaccines are in use and in the pipeline (1). In general, the evidence suggests that the efficacy of the vaccines is in the prevention of severe symptomatic disease, but there is limited evidence about the durability of the immune protection (2). It has been described that after natural infection, the immunological memory remains at least for 6 months (3).
Healthcare workers (HCWs) all over the world were prioritized for the vaccination because of the high risk of infection and the well-known impact on the health workforce (4–6). Gomez Ochoa et al. in a systematic review and meta-analysis reported 11% of HCW with COVID-19 and 5% developed severe disease (5). Additional consequences were observed in psychological well-being and staff retention among others.
Vaccination becomes a highly effective measure for minimizing the adverse consequences of the disease. Nevertheless, post-vaccination SARS-CoV-2 infections were observed and probably related to multiple factors, including the variable vaccine efficacy and the emergence of new viral variants (7–10).
In a COVID-19 dedicated facility in Western Qatar, the vaccination program of 930 HCWs (physician, nurses, and technologists) started in December 2020 with either Pfizer or Moderna vaccine. The surveillance of SARS-CoV-2 infection in HCWs, with data kept after vaccination, included daily temperature monitoring, reporting of symptoms, and contact tracing of exposures, and a monthly mandatory SARS-CoV-2 polymerase-chain reaction (PCR) or antigen test. Positive antigen test results were confirmed by PCR. During the period from December 2020 to August 2021, 16 vaccinated HCWs with COVID-19 were reported. The cases are described in this report.
Case presentation
Out of 930 HCWs (physicians, nurses, and technologists) vaccinated during the period from December 2020 to August 2021, COVID-19 was confirmed by positive PCR post-vaccination in 16 cases (1.7%): 2 male and 14 female cases. All cases received the primary vaccination (two doses). The median age was 45.5 years (minimum 29, maximum 60, and interquartile range 14.5). Five cases had a previous history of chronic conditions, mainly hypertension (HBP) and diabetes mellitus (Table 1). During the study period, the Delta and Alpha variants of SARS-CoV-2 were predominant in the country.
The cases were diagnosed because of the periodic lab test done in asymptomatic cases (6 HCW) or symptoms (10 cases). The most frequent symptoms at the time of diagnosis were loss of smell (four cases), cough (four cases), fever (two cases), nasal discharge or obstruction (three cases), general malaise (two cases), and dyspnea and loss of taste in one case each.
The median time between the second dose of primary vaccination and the positive PCR was 132.5 days (minimum 59, maximum 211, and interquartile range 91). The median cycle threshold (CT) value at the time of diagnosis was 25.1 (minimum 17.3, maximum 35.4, and interquartile range 7.3). After 10 days of the initial positive PCR, the test became negative or inconclusive in nine cases and reactive (CT ≥ 30) in five cases, and in two cases, the values were positive (CT values are 27.86 and 24.16).
Laboratory tests performed at diagnosis showed results mostly in normal parameters, including white blood cell count (median 6.4 × 103/L; minimum-maximum: 3.7-11.6), absolute neutrophil count (3.5 × 103/L; 0.6–8.7), lymphocyte count (1.8 × 103 × 103/L; 1.0–2.7), serum creatinine (59 µmol/L; 34–91), alanine transaminase (17.5 U/L; 10–182), aspartate transaminase (17.5 U/L; 10–89), and lactate dehydrogenase (167.5 U/L; 120–274). D-dimer (0.3 mg/L; 0.2–4.4) was elevated in five cases.
In six cases, the chest X-ray did not show pulmonary findings; in three cases, ground glass images were observed; in six cases, several other lesions (interstitial and inflammatory) were noted; and in one case, the combination of various findings suggested of COVID-19 (case no. 9). Figure 1 shows the maximum oral temperature with descending values during the first 10 days after confirmation, without differences between cases with/without antibiotics.
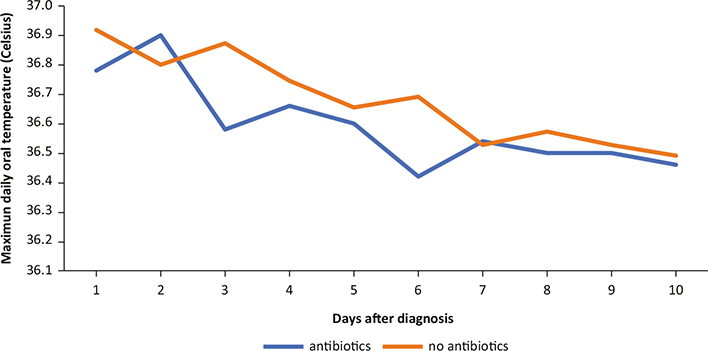
Fig. 1. Median daily maximum oral temperature according to antibiotic use in postvaccination COVID-19 (Celsius degree).
The most frequent treatment was an antiviral (favipiravir) (13 cases), and antibiotics (amoxicillin-clavulanic acid or azithromycin) were prescribed in five cases. The clinical course of the disease was satisfactory, with no complications or sequelae at discharge.
Discussion
Regardless of the number of HCWs confirmed with COVID-19 after vaccination, which could be an expression of immune system failure, the clinical picture suggests a more benign disease in comparison with non-vaccinated SARS-CoV-2 infections. The current protocol recommends the use of antiviral drugs, but the requirement of antibiotics, usually prescribed as an empirical and prophylactic approach, could be questioned. The improper use of antibiotics is related to the development of antimicrobial resistance, and the risk of associated healthcare-associated infections adds a significant impact on the cost of healthcare, limited by the global economic crisis generated by the pandemic.
Disease severity changes should be considered an added value of the vaccination. Various papers published report of the findings of mild disease, the limited requirement of medical care, and easy recovery in COVID-19 in HCW after vaccination (7, 8, 10).
In addition, from the infection control point of view, it is important to maintain the staff monitoring system using various methods, involving monitoring of respiratory symptoms and lab tests when necessary, and the requirement of vaccination for the newly hired non-vaccinated staff (and booster doses for those with previous full vaccination). In order to address patient and staff safety, the respiratory protection program, the recommendations for isolation precautions, and the use of personal protective equipment should be reviewed according to the new environments generated by the pandemic.
This report provides evidence, suggesting that COVID-19 after vaccination of HCWs is a mild disease with a good prognosis. Moreover, there is need to strengthen the infection control program focused on patient and staff safety and transmission prevention in healthcare facilities.
Acknowledgment
The authors would like to acknowledge Alexis Gonzalez Velázquez for the proofreading of this manuscript.
Ethics statement
The research was exempt from ethical approval because of the involvement of the collection of existing data, with data protection measures using identifiers linked to the medical records of the staff.
References
| 1. | Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022; 28(2): 202–21. doi: 10.1016/j.cmi.2021.10.005 |
| 2. | Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021 Jan 7; 384(1): 80–2. doi: 10.1056/NEJMc2032195 |
| 3. | Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021 Feb 5; 371(6529): eabf4063. doi: 10.1126/science.abf4063 |
| 4. | Bandyopadhyay S, Baticulon RE, Kadhum M, Alser M, Ojuka DK, Badereddin Y, et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health 2020 Dec; 5(12): e003097. doi: 10.1136/bmjgh-2020-003097 |
| 5. | Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021 Jan 4; 190(1): 161–75. doi: 10.1093/aje/kwaa191 |
| 6. | Maltezou HC. Vaccination of healthcare personnel in the COVID-19 era: a call for actions. Vaccine 2021; 39(51): 7363–5. doi: 10.1016/j.vaccine.2021.10.078 |
| 7. | Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers – eight U.S. locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021 Apr 2; 70(13): 495–500. doi: 10.15585/mmwr.mm7013e3 |
| 8. | Teran RA, Walblay KA, Shane EL, Xydis S, Gretsch S, Gagner A, et al. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members – Chicago, Illinois, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021 Apr 30; 70(17): 632–8. doi: 10.15585/mmwr.mm7017e1 |
| 9. | Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley RT, Currier JS, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med 2021 May 6; 384(18): 1774–5. doi: 10.1056/NEJMc2101927 |
| 10. | Fageeh H, Alshehri A, Fageeh H, Bizzoca ME, Lo Muzio L, Quadri MFA. Re-infection of SARS-CoV-2: a case in a young dental healthcare worker. J Infect Public Health 2021 Jun; 14(6): 685–8. doi: 10.1016/j.jiph.2021.02.012 |