ORIGINAL ARTICLE
Seroprevalence in Argentinian healthcare workers after vaccination with Sputnik V
Santonato Daniela1*#, María A Malvicini1, Andrea Novau1, Silvio F Torres2, Alejandro Siaba Serrate2, María V Romano3, Pablo G Brenzoni3, Leonardo Fabbro1, Laura Paulosky1 and Wanda Cornistein1
1Infection Control Department, Hospital Universitario Austral, Universidad Austral, Pilar, Buenos Aires, Argentina;
2Paediatric Intensive Care Unit, Hospital Universitario Austral, Universidad Austral, Pilar, Buenos Aires, Argentina;
3Central Laboratory, Hospital Universitario Austral, Universidad Austral, Pilar, Buenos Aires, Argentina
Abstract
Background: Healthcare workers (HCW) were deeply affected by coronavirus disease 2019 (COVID-19). Therefore, vaccination of this population is crucial. However, data on Sputnik V vaccine are sparse.
Objective: The aim of this study was to evaluate serological responses in HCWs following two doses of Sputnik V vaccine.
Methods: A cross-sectional study was conducted at a tertiary-care private teaching hospital between April and May 2021. HCWs without a history of COVID-19 3 or more weeks after the second dose of Sputnik V had a fresh serum sample extracted and processed using Abbott® SARS-CoV-2 IgG II Quant. Values equal to or over 50 arbitrary units (AU)/mL were considered positive. Primary outcome was the proportion of participants who developed antibodies 21 or more days after the second dose of Sputnik V. Secondary outcomes were concentration of anti-spike IgG antibodies and comparison of such concentrations between samples taken 3–5 weeks and more than 5 weeks after the second dose.
Results: The entire population developed anti-spike IgG antibodies. The median antibody concentration was 1234.8 AU/mL. When analysing days to extraction from second vaccine dose, there was no statistical difference between 21 and 35 days versus more than 35 days.
Conclusion: Vaccination with Sputnik V in HCW at our institution demonstrated an efficacy of 100% in achieving quantifiable anti-spike IgG antibodies 21 or more days after the second dose.
Keywords: COVID-19; vaccination; antibodies; occupational health; healthcare workers; Argentina
Citation: Int J Infect Control 2022, 18: 21791 – http://dx.doi.org/10.3396/ijic.v18.21791
Copyright: © 2022 Santonato Daniela et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 7 July 2021; Revised: 29 December 2021; Accepted: 6 March 2022; Published: 27 May 2022
Competing interests and funding: The authors have no relevant financial or non-financial interests to disclose. They did not receive support from any organization for the submitted work.
*Santonato Daniela, Infection Control Department, Hospital Universitario Austral, 1500 Presidente Juan Domingo Perón Avenue, Pilar, Buenos Aires Province, Argentina. Email: daniela.santonato@gmail.com
The coronavirus disease 2019 (COVID-19) pandemic has had a significant impact on people’s lives. Since its first outbreak in December 2019 in Wuhan, China, the disease has spread across the globe, affecting 173,386,978 individuals and reporting over 3,730,939 deaths by June 7, 2021 (1).
Healthcare workers (HCWs), as frontline personnel, were deeply affected by the infection early in the pandemic. According to the epidemiological update on COVID-19 from the Panamerican Health Organization at the time of this study, HCW accounted for 2.46% of the cases and 0.55% of deaths in America between January 2020 and November 2021 (2). A report by the Centers for Disease Control and Prevention (CDC) (3) stated that in the United States between February 12 and July 16, 2021, 1.5% of COVID-19 cases occurred in HCWs. In Argentina, data are sparse. However, by June 29, 2020, 9% of COVID-19 cases in Buenos Aires province corresponded to HCWs (4).
In Argentina, the first vaccine that was granted authorization by the National Administration of Medicine, Food and Medical Technology (ANMAT) was Sputnik V, from the Gamaleya National Research Centre for Epidemiology and Microbiology, Russia. The vaccine uses non-replicating human adenoviruses as vectors, rAD26 as the first component and rAD5 as the second component, both of which carry the gene for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) full-length glycoprotein S (rAd26-S and rAd5-S) (6). rAd26-S and rAd5-S are administered intramuscularly separately with a minimum interval of 21 days.
Given the limited supply of vaccines, the government adopted a staggered approach. Among the first beneficiaries of the campaign, which began on December 29, 2020, were HCWs. Soon thereafter, however, the government opted to extend dose intervals for COVID-19 vaccines in order to optimize early vaccine rollout and population protection in the case of limited vaccine supply. This affected primarily those who received a first dose of Covishield (Serum Institute of India, Pune, India) or Sinopharm (China National Pharmaceutical Group Corporation, Beijing, China), given that the rollout of these vaccines started in late February. Nonetheless, there was a shortage of Sputnik V’s second component from April to June with the same effect in terms of dose interval.
Published studies (6–10) prior to ours reported on the efficacy of COVID-19 vaccines. The vaccines currently approved for human use have demonstrated a high efficacy against hospitalization and death from COVID-19. Nonetheless, the efficacy against mild and moderate disease is variable. In this respect, the interim analysis of the phase-3 randomized controlled study on Sputnik V showed an overall efficacy of 91.6% (6). A preprint article evaluated the sera of a cohort of 12 recipients of Sputnik V against variants of concern (VOC) of interest. The authors determined a concerning potential of VOC to escape the neutralizing antibody responses that Sputnik V elicits (11).
VOCs are novel SARS-CoV-2 lineages for which there is evidence of an increase in transmissibility, more severe disease (e.g. increased hospitalizations or deaths), and significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures (12). There is a growing body of evidence that people who have been vaccinated, or even had the disease, can contract COVID-19 (13, 14). What is more, it is estimated that 8–10% of fully vaccinated people do not create antibodies.
A study by the Health Ministry of Buenos Aires province and the Ministry of Science, Technology and Innovation (15) studied the immune response of HCW after vaccination with Sputnik V. The authors found that 89% (126/142) of the study population developed anti-spike IgG antibodies 21 days after the first dose, and 100% (142/142) developed said antibodies 21 days after the second dose. The results of the study were published on the National Government’s official website, while our study was ongoing.
To our knowledge, none of the articles aimed at investigating humoral responses in HCWs after COVID-19 vaccination in Argentina have been published in an indexed Journal. What is more, there has been controversy surrounding the veracity of evidence presented by the Gamaleya National Institute. We sought to conduct a study at our institution in order to evaluate serological responses in HCWs following two doses of Sputnik V and their behaviour in relation to time.
Methods
Study design and setting
This was a cross-sectional study conducted between April and May 2021 at a tertiary care private teaching hospital in Buenos Aires province. The institution has over 3,100 HCWs, with 19.88% of them contracting the disease as of June 3, 2021.
The government runs the vaccination campaign. Nonetheless, the Department of Infection Control and Prevention, together with the Department of Infectious Diseases, has incentivized the personnel to get vaccinated through written communications and virtual gatherings aimed at answering questions and dispelling myths surrounding vaccination.
Since its beginning in late December 2020 until June 2021, over 80% of the payroll has received at least one dose of vaccine and 29% two doses. Among the latter, 11.8% had COVID-19 prior to vaccination.
Participants
During the first 6 weeks of the study, we included HCWs 3–5 weeks after the second dose of Sputnik V. Thereafter, the Institutional Review Board (IRB) approved an amendment to extend the inclusion criteria to HCW 3 or more weeks after the second vaccine dose. We excluded participants with history of COVID-19 defined by a positive reverse transcriptase polymerase chain reaction (RT-PCR) test, positive IgG before vaccination, or symptoms without evaluation. All participants provided signed informed consent to be included in the database for participation.
Procedure
Participants included in the study had a blood sample collected by venipuncture. The sample was processed using Abbott® SARS-CoV-2 IgG II Quant (Abbott Diagnostics, Buenos Aires, Argentina) following the manufacturer’s instructions. The chemiluminescent microparticle immunoassay (CMIA) was used for the qualitative and quantitative determination of IgG antibodies to SARS-CoV-2, specifically antibodies against the receptor binding domain (RBD) in fresh human serum on the Alinity i System. Quantitative results reported by the instrument were used in the analyses. As per manufacturer’s instructions, a value equal to or over 50 arbitrary units (AU)/mL was considered positive.
Statistical analysis
Continuous data were expressed as mean and median ± standard deviation (SD), including interquartile range (IQR). We performed an analysis of antibody concentration through box-plot and scatter plot. We estimated mean and SD for the measures of central tendency and dispersion, observing outliers in the concentration of antibodies.
Furthermore, we analysed differences in antibody concentrations in relation to the time of extraction of serum sample from the second vaccine dose. For this, the cohort was divided in two groups according to the testing time from the second vaccine dose (Group 1 3–5 weeks; Group 2 more than 5 weeks). Given the non-normal distribution of both populations, a Wilcoxon rank-sum (Mann–Whitney) test was performed. We employed STATA10.0 software (StataCorp LLC, College Station, TX, USA).
Outcomes
The primary outcome was the proportion of participants who developed antibodies 21 or more days after the second dose of Sputnik V. The secondary outcomes include concentration of anti-RBD IgG antibodies in the participants and comparison of such concentrations between participants who had the serum sample taken 3–5 weeks after the second dose, and those whose antibody concentration was determined more than 5 weeks after the second dose.
Results
Between April and May 2021, 186 HCW were eligible and signed an informed consent to participate in the study protocol. Twenty-three participants were excluded from the analysis (Fig. 1). The characteristics of the cohort are included in Table 1. All of the participants developed anti-RBD IgG with a mean concentration of 2359.501 AU/mL (SD: 3109.585; IQR: 2,330). When analysing the concentration of antibodies in relation to time from the second vaccine dose, there was no statistical difference between both groups (P = 0.0897; Fig. 2).
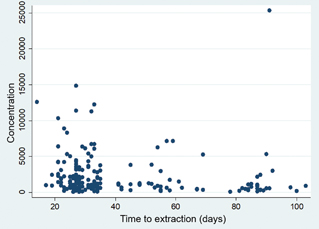
Fig. 2. Concentration of antibodies versus time from the second vaccine dose.
Concentration is in arbitrary units/mL.
Discussion
Data from the literature suggest that the Sputnik V vaccine has an efficacy of 91.6% (6). In a previous study by the Health Ministry of Buenos Aires Province and the Ministry of Science, Technology and Innovation (15), the authors found that 100% of the participants developed anti-spike IgG antibodies 21 days after the second dose of the vaccine.
In this study, we were able to corroborate these findings. The totality of the cohort had anti-RBD IgG antibodies over the threshold. We believe this is of great importance, given that HCWs are continuously exposed to the disease and, what is more, if infected they can pose as a source of infection for healthy patients.
It is still uncertain how long humoral immunity lasts following vaccination. In this study, we were able to observe that the concentration of antibodies did not differ significantly between samples obtained 21–5 days from the second vaccine dose and those extracted more than 35 days from the said dose. Going forward, the study cohort will be followed prospectively in order to identify vaccine breakthrough infections and antibody concentrations through time.
This study has its limitations. First, we did not assess baseline serological status before vaccination began. Nonetheless, by excluding those with confirmed COVID-19 or symptoms without medical evaluation only those with asymptomatic infection would have been included in the study. Second, the study included only one serological determination after complete vaccination and, hence, we were not able to estimate how long the immunity lasts. However, we are currently working on the second part of the study in which participants will have a serological test 6 months after the second dose. Third, the test utilized in this study, albeit its quantitative determination, is a CMIA and does not have an established correlation with the presence and quantity of neutralizing antibodies. We aim to establish such correlation in our future study. Finally, we did not address cellular immunity, which has been found to play an important role in the defence against SARS-CoV-2.
In conclusion, vaccination with Sputnik V in HCWs at our institution has demonstrated an efficacy of 100% in achieving quantifiable anti-spike IgG antibodies 21 or more days after the second dose. Further studies are needed to determine whether the concentration of antibodies will remain steady with time, as well as to establish the concentration of IgG that is effectively correlated with the presence of neutralizing antibodies.
Acknowledgements
The authors thank the Infectious Diseases Department at Hospital Universitario Austral for participating in the recruitment phase of the study.
Ethics approval
Approval was obtained from the Institutional Review Board at Hospital Universitario Austral, Universidad Austral. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
References
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available from: https://coronavirus.jhu.edu/map.html [cited 7 June 2021].
- Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización epidemiológica: Enfermedad por Coronavirus (COVID-19). 2 de diciembre de 2021, Washington, D.C.: OPS/OMS; 2021. Accessed December 26, 2021. Available at https://www.paho.org/es/documentos/actualizacion-epidemiologica-enfermedad-por-coronavirus-covid-19-2-diciembre-202.
- Hughes MM, Groenewold MR, Lessem SE, Xu K, Ussery EN, Wiegand RE, et al. Update: characteristics of health care personnel with COVID-19 – United States, February 12–July 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1364–8. doi: 10.15585/mmwr.mm6938a3
- Ministerio de Salud. Provincia de Buenos Aires. Informe de situación del personal de salud al 29 Jun 2020. La Plata: Dirección Provincial de Hospitales; 2020.
- Logunov D, Dolzhikova I, Shcheblyakov D, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021; 397: 671–81. doi: 10.1016/S0140-6736(21)00234-8
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19 [published online ahead of print, 2021 Apr 21]. N Engl J Med 2021; 384: 2187–201. doi: 10.1056/NEJMoa2101544
- Baden LR, El Sahly H, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021; 384: 403–16. doi: 10.1056/NEJMoa2035389
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–15. doi: 10.1056/NEJMoa2034577
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [published correction appears in Lancet 2021 Jan 9; 397: 98]. Lancet 2021; 397: 99–111. doi: 10.1016/S0140-6736(20)32661-1
- Ikegame S, Siddiquey MNA, Hung CT, Haas G, Brambilla L, Ogutuyo KY, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Version 3. medRxiv. Preprint. NaN NaN [revised 2021 May 29]. doi: 10.1101/2021.03.31.21254660
- Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions. 2021. Accessed December 26, 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- Yahav D, Yelin D, Eckerle I, Eberhardt CS, Wang J, Cao B, Kaiser L, et al. Definitions for COVID-19 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect 2021; 27: 315–8. doi: 10.1016/j.cmi.2020.11.028
- Birhane M, Bressler S, Chang G, Clark T, Dorough L, Fischer M, et al. COVID-19 vaccine breakthrough infections reported to CDC – United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 792–3. doi: 10.15585/mmwr.mm7021e3
- Empleo de la vacuna Sputnik V en Argentina: Evaluación de respuesta humoral frente a la vacunación Informe parcial Enero-Marzo 2021. Accessed December 26, 2021. Available from: https://www.argentina.gob.ar/sites/default/files/informe_sputnik_buenos_aires_3.03.2021v1.pdf.
