PRACTICE FORUM
Development and operation of an indoor mass COVID-19 vaccination clinic
Ivan W. Gowe1*, David Crist1, Brittany Kiteley1, Caitlin Owens1 and Adrienne Giddens1
UNC Pardee Hospital, 800 North Justice St., Hendersonville, NC 28791
Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, many public response activities were conducted outdoors to reduce the risk of transmission. This was in adherence to existing infection prevention recommendations. We report the development and safe, efficient operation of a mass COVID-19 vaccination clinic during a time when guidelines for indoor clinics were limited.
Keywords: COVID-19; COVID-19 vaccines; vaccination; community; United States
Citation: Int J Infect Control 2021, 17: 21590 – http://dx.doi.org/10.3396/ijic.v17.21590
Copyright: © 2021 Ivan W. Gowe et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 7 May 2021; Revised: 15 September 2021; Accepted: 22 October 2021; Published: 17 December 2021
Competing interests and funding: The authors declare no conflicts of interest. No funding was received for this initiative.
*Ivan W. Gowe, Email: ivan.gowe@unchealth.unc.edu
In March 2020, our hospital collaborated with our county health department and a local community college to deploy a mass coronavirus disease 2019 (COVID-19) testing facility. Given the paucity of information on disease transmission, we performed testing in tents assembled in a parking lot. For the mass vaccination clinic, an outdoor location was not feasible primarily because of the weather. Armed with more information on the spread of COVID-19 and access to personal protective equipment, we describe here our safe development and operation of an indoor mass vaccination clinic.
Objective
Pardee Hospital serves almost 120,000 county residents in Western North Carolina. About 26% of the county’s residents are older than 65 years of age (1). We registered the facility as a community vaccine provider with the North Carolina Department of Health and Human Services (NC DHHS) in September 2020. The space we used for staff vaccination was too small for community vaccinations. We considered the development of an outdoor clinic to reduce infection transmission risk by respiratory droplet dilution and dispersion. However, we decided it would not be safe to manage patients over 65 years of age outside in winter weather. Additionally, the logistics of using outdoor tents (i.e. heating, power, internet connectivity, vaccine temperature control, etc.) precluded us from their use.
Design
We collaborated with a local community college and opened the first mass vaccination site in our region in a 7,500 square foot auditorium. The college provided parking space, the auditorium, furniture, and staff (including nursing students and faculty). The county Emergency Management team provided emergency medical equipment and navigated state support. We collaborated with the local health department in planning; though they focused on opening more vaccination centres in the community. The hospital’s Process Innovation (PI) team led the logistical development of the clinic in collaboration with Infection Prevention, Pharmacy, Emergency Management, Occupational Health, and many other departments. The PI team gathered and organised information from the various departments and project managed the whole venture in a way that allowed hospital department heads to return to pandemic management during a time (November 2020–January 2021) with the highest COVID-19 patient census.
Once the process flow was finalised, staff training materials were developed and deployed. Every clinical role had a competency checklist, which was completed before the initiation of work. Every non-clinical role had standard work to follow. Pharmacy staff training included vaccine storage requirements, training for drawing up the vaccine, and syringe labelling.
Vaccine process flow
The state arranged for vaccine allocations to be delivered to the hospital for storage in an ultra-low freezer until the day of use. Upon receipt, the vaccine was logged into an inventory management system. The Pharmacy team transported the vaccine to and from the clinic. They stored the vaccine vials in a temperature-monitored refrigerator at the college. The doses from each vial were drawn up and placed in a light protection bag. The bag was labelled with the lot number, dilution time, and an expiration time for that vial. Pharmacy staff also created labels for the individual syringes. These labels were placed on the patient’s vaccine card. Using an adapted kitchen, vaccines were drawn up, diluted, and prepared for administration to correspond with each appointment time. Any wasted vaccine was recorded; at the time of submission, we had wasted 0.4% of doses for various reasons, including dropped syringes, bent needles, failed syringes, and the vast majority due to missed appointments. The vaccination clinic managed all three vaccines approved in the United States (US) at the time – two dose Pfizer-BioNTech (BNT162b2), two dose Moderna (mRNA-1273), and the single dose Johnson & Johnson Janssen (JNJ-78436735).
Patient process flow
The patient process was designed to minimise queuing and bottlenecks, maximise physical distancing, and achieve a uni-directional flow to reduce the risk of COVID-19 transmission. The safety of the patients and staff was maintained in higher regard than efficiency. This was accomplished using Takt time studies and models based on the employee vaccination clinic flow. The benefit of an auditorium was that we were not constrained by existing walls, features, etc. This final setup is shown in Fig. 1.
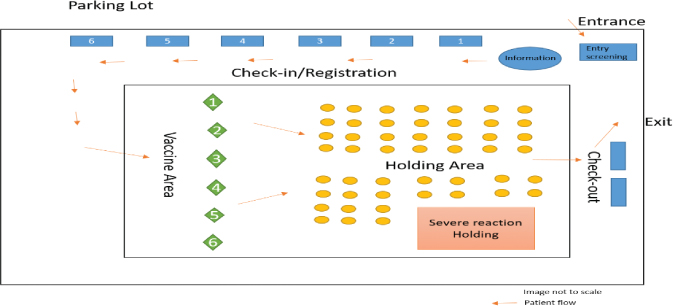
Fig. 1. Schematic of indoor vaccine clinic setup.
The process followed comprised the following steps:
All patients registered on the state’s vaccine website or through a call centre and received an appointment.
Patients presenting for their vaccine appointments were directed by signage to stay in their vehicle until their appointment time, to reduce queuing and crowding. A local golf club donated carts and drivers to assist patients to and from their cars. Wheelchairs were available for patient use.
Entry screening: A sign at the entrance showed COVID-19 symptoms and screening questions. Patients were screened and given a mask if they did not have one. Patients could wear their own masks as long as they were visibly clean and in good repair. Patients with COVID symptoms were rescheduled and their appointment was offered to someone on a waiting list.
Check-in/registration: Patients progressed to the registration tables where their vital information was input to an electronic medical record (EMR). They signed required documents, and received copies of the emergency use authorisation and vaccine safety information.
Vaccine area: A volunteer directed patients to the next available vaccination booth. Patient identifiers were verified and vaccine was administered, and documented in the EMR and on a vaccination record card for the patient. Patients received a card with their vaccination time. After the vaccinator and scribe completed cleaning the space, they changed an indicator flag from red to green.
Holding area: An observation area was designed to be large enough to prevent bottlenecks. We placed pairs of chairs (to accommodate a vaccinee and a household member) at least six feet apart. Nurses monitored patients for 15 min and finalised patient documentation on a mobile computer. The observation started at the time written on the vaccination time card. Another staff member scheduled appointments for the second vaccine and wrote it on the vaccination record card. Patients with a history of vaccine reactions were monitored for 30 min. Space was allocated to manage patients with active severe reactions. While vaccinees waited, repeating slides showed answers to frequently asked questions and provided sources for more information on COVID-19 and the vaccine. The observation period was the largest delay in this process so the PI team put as much activity into this time to reduce crowding and queuing.
Check-out: The holding area nurse released patients from the observation area. As the patients exited, the scheduling of their second appointment was verified.
Exit: Patients leaving the building could walk or ride the golf cart back to their vehicles.
Infection control processes
Based on guidance from the state health department (NC DHHS) and United States (US) Centers for Disease Control and Prevention (CDC), our primary emphasis was to ensure patients and staff were always masked and maintained physical distance (2, 3). All staff wore eye protection in keeping with CDC guidelines (4). In addition, high touch items like handrails, pens, vaccination tables, chairs, doorknobs, etc. were disinfected. The US Environmental Protection Agency (EPA) released a list (List N) of disinfectants that were shown to be active against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or similar viruses. We ensured our cleaning products were on that list. Alcohol-based hand rub was available at every station for staff and patients. NC DHHS developed a slogan that emphasised ‘three Ws’ of COVID prevention – wear a face covering, wash your hands, and wait at least six feet away from others (2). We posted signs to remind patients of these prevention measures throughout the building. We placed markers on the floor so everyone was at least six feet away from non-family members. Clinic staff reminded patients of all these measures.
The CDC defined close contact as being within 6 feet of an infected person for more than 15 min over 24 h (3). The patient flow was designed to avoid that length of exposure. The highest risk time was during the observation period. Chairs were over 6 feet apart and the staff was trained to leave empty chairs between patients to reduce the risk of close contact and exposure.
Results
At the peak of their capacity, the clinic was administering up to 900 vaccines per day. After 33 days, the clinic celebrated its 10,000th dose. At the time of submission, the vaccine clinic had administered 23,512 COVID-19 vaccines. This made up 47% of the vaccines administered in Henderson County.
Personnel response
The vaccine clinic depended on staff working overtime, and on leaders releasing staff from their regular schedule to work in the clinic. Staff and community members with relevant experience or training were asked to train and be checked off to work at the clinic. Both the hospital staff and the community volunteers responded very positively to the opportunity.
Discussion
It makes sense from what we know that outdoor spaces would be safer for mass COVID-19 vaccination or testing (5). However, outdoor spaces are not always ideal due to weather, availability of information technology infrastructure, storage of temperature-sensitive vaccines, frail patients, and other reasons. There are prevention measures that can be instituted to mitigate the risk of transmission indoors, the primary ones being strict masking compliance and physical distancing. The importance of masking to prevent COVID-19 transmission has been well reported in the literature (6, 7). The key value of the building used was its size. We were able to keep many people separated by more than 6 feet. Operationally, we accomplished this by reducing queuing. This had the added benefit of ensuring that the CDC definition of close contact was not met. We were, therefore, able to vaccinate a lot of our community safely and efficiently. We did not vaccinate patients in their vehicles. This would have been convenient for some, but would have necessitated the development of two separate processes. We did not have the staff to provide that service.
Conclusion
Here we describe the development of an indoor mass COVID-19 vaccine clinic. This project provided free vaccines to over 23,000 residents of Henderson and surrounding counties in Western North Carolina. Vaccine clinics do not have to be staged outside in the elements when source control and physical distancing can be maintained. This was done by ensuring everyone was masked, minimising queues, and maximising space between people.
Acknowledgements
Many thanks for the involvement and assistance from Pardee Hospital, Blue Ridge Community College, Henderson County Sheriffs, Kenmure Golf Course, Henderson County Emergency management.
Ethical approval
Ethical approval was not required.
References
- Radcliff A, Peterson J, Champion M, Compher J. Henderson county annual profile of statistical information. 2020. Available from: https://www.hendersoncountync.gov/sites/default/files/fileattachments/planning/page/5891/stat_brochure_2020.pdf [cited 15 September 2021].
- Know Your Ws: Wear, Wait, Wash. North Carolina Department of Health and Human Services; 2020. Available from: https://covid19.ncdhhs.gov/slow-spread/know-your-ws-wear-wait-wash [cited 15 September 2021].
- Centers for Disease Control and Prevention. Appendices. Atlanta, GA: Centers for Disease Control and Prevention; 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html#contact [cited 15 September 2021].
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Atlanta, GA: Centers for Disease Control and Prevention; 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [cited 15 September 2021].
- Centers for Disease Control and Prevention. Participate in outdoor and indoor activities. Atlanta. GA: Centers for Disease Control and Prevention; 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/outdoor-activities.html [cited 19 August 2021].
- Honein MA, Christie A, Rose DA, Brooks DA, Meaney-Delman D, Cohn A, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. MMWR Morb Mort Wkly Rep 2020; 69(49): 1860–7. doi: 10.15585/mmwr.mm6949e2
- Gandhi M, Marr LC. Uniting infectious disease and physical science principles on the importance of face masks for COVID-19. Med (NY) 2021; 2(1): 29–32. doi: 10.1016/j.medj.2020.12.008