ORIGINAL ARTICLE
Unprepared and unprotected: Graduating medical students’ knowledge, attitudes, and practices regarding drug-resistant tuberculosis in Cape Town, South Africa
Michael J. Harrison1*, Johnathan Watts1, Michael-Jon Rosslee1, Arne von Delft1,2 and Helene-Mari van der Westhuizen2,3
1School of Public Health and Family Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa; 2TB Proof, Cape Town, South Africa; 3Nuffield Department of Primary Health Care Sciences, University of Oxford, Oxford, United Kingdom
Abstract
South Africa has a high burden of drug-resistant tuberculosis (DR-TB), which has a particularly high mortality among healthcare workers. Junior clinicians deliver key DR-TB services and require training in DR-TB management and prevention. This study aimed to investigate graduating medical students’ knowledge, attitudes, and practices relating to DR-TB, including management, infection control measures, and occupational health services. This cross-sectional, questionnaire-based study at the University of Cape Town, South Africa, recruited final year medical students and included 87 participants. The mean DR-TB knowledge score was 4.7 points (95% confidence interval [CI]: 4.42–5.06, maximum score 8 points). Students reported challenges in accessing respiratory protection, with half (47.7%) struggling to find an N95 respirator when needed. DR-TB exposure was reportedly common. Three students reported prior TB disease, approximately half (n = 49, 55.9%) reported personal concern of active DR-TB disease during undergraduate studies, and the majority (n = 80, 91.9%) correctly perceived themselves to be at increased risk compared to the general population. Medical students are currently unprepared for their role in managing DR-TB in South Africa and unprotected against occupational illness during their studies. This should be addressed in undergraduate curricula and in establishing comprehensive occupational health policies. Resilient personal protective equipment (PPE) supply chains, infection control training, and comprehensive occupational health support have relevance to both DR-TB and novel pathogens, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Keywords: tuberculosis; drug-resistance; occupational diseases; healthcare workers; safety; medical education; infection control; personal protective equipment; South Africa
Citation: Int J Infect Control 2021, 17: 21110 – http://dx.doi.org/10.3396/ijic.v17.21110
Copyright: © 2021 Michael J. Harrison et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 12 December 2020; Revised: 23 March 2021; Accepted: 23 April 2021
Competing interests and funding: Drs von Delft and van der Westhuizen were involved in undergraduate TB infection control and occupational health training through their volunteer work for TB Proof. Neither of them received any remuneration for their involvement in this training. The lead authors (MH, JW, MJR) have no competing interests to declare. The authors received no funding for this work.
*Michael J. Harrison, Faculty of Health Sciences, University of Cape Town, Anzio Road, Observatory, Cape Town, 7935, South Africa. Email: michael.john.thomas.harrison@gmail.com
To access the supplementary file, please visit the article landing page
Mycobacterium tuberculosis has claimed more lives than any other infectious disease, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a negative impact on tuberculosis (TB) prevention and care (5). In South Africa, the annual TB incidence is estimated at 615 cases per 100,000 people, with more than 14,000 cases being resistant to first-line therapy annually (5).
South Africa has been pioneering a community-based approach to DR-TB care, whereby patients are diagnosed and initiated on appropriate therapy at their primary care clinic or district hospital (6, 7). This decentralized care structure requires HCWs to have sufficient knowledge and competencies to effectively manage DR-TB, including junior doctors in their initial 3 years of medical practice.
Managing DR-TB correctly is important not only in the provision of care but also for HCW safety, as patients who are on effective treatment pose less risk of ongoing transmission of DR-TB (8). Knowledge deficits may negatively influence HCWs’ attitudes and practices, undermining patient care, and potentially contribute to DR-TB transmission within healthcare facilities (7, 9, 10). South African HCWs are reported to have at least a three-fold risk of drug-sensitive TB (DS-TB) and a six-fold risk of DR-TB relative to the general population (11–15). Several studies from high DR-TB prevalence countries have reported increasing awareness of personal DR-TB risk among HCWs (16, 17); however, few have investigated behavioral responses to perceived risk.
Undergraduate medical students also suffer considerable rates of occupational TB, including extra-pulmonary and drug-resistant disease (18). Currently, South African students are excluded from the policies that protect HCWs and have no recourse to compensation for diagnostic and treatment costs, loss of career and academic opportunities, and temporary or permanent disability (18, 19). This makes students vulnerable to occupational TB, a dynamic that may negatively affect the way they treat DR-TB patients.
Several studies have reported health workers having insufficient knowledge, stigmatizing attitudes, and inappropriate practices relating to DS-TB management and prevention in TB-endemic regions, including South Africa (20–28). However, few studies have considered DR-TB, and none have focused on the role of junior clinicians. This study aimed to investigate graduating medical students’ knowledge, attitudes, and practices relating to DR-TB, including DR-TB care, infection control measures, and occupational health services.
Methods
This study was conducted at the University of Cape Town (UCT) in Cape Town, South Africa, and was led by senior undergraduate medical students. It was designed in response to encounters with DR-TB patients during training and their anticipated role in managing DR-TB patients after graduation. Ethical approval and institutional permission were obtained from the UCT Human Research Ethics Committee (HREC 582/2018).
Study participants
All individuals enrolled in the graduating class of the Bachelor of Medicine and Bachelor of Surgery (MBChB) degree program at UCT in 2018 were approached through an online invitation to participate in this questionnaire-based cross-sectional study (N = 240). Medical students were sampled 2 months prior to graduation and placement at facilities across South Africa as medical interns. Their responses provide an indication of the undergraduate training in DR-TB care they received. In South Africa, undergraduate medical training takes place primarily within a clinical environment, and students interact with patients and engage in clinical work on a daily basis during the last 4 years of their degree.
Questionnaire design and administration
A questionnaire (Supplementary Material) was designed specifically for this study as existing tools were aimed at different HCW roles, or students from various degree programs, and did not focus on DR-TB (20, 22, 23, 30). We collated previously published questionnaires and generated new questions based on provincial clinical guidelines and two review articles (31–33). The questionnaire was reviewed for face validity by professionals working in TB infection control, piloted in a group of pre-final year medical students, and refined based on feedback.
Statistical analysis
Data were anonymized prior to analysis. Characterization of participants’ knowledge, attitudes, and practices was summarized using descriptive statistics. Self-reported and observed knowledge scores were compared, as were health-seeking behaviour and access to private healthcare.
Where appropriate, the chi-squared test and Spearman’s rank correlation were used. All data were analyzed through the statistical software package R version 3.4.1 (The R-Roundation for Statistical Computing, https://www.r-project.org)
Results
The questionnaire was completed by 87 final year medical students (36.3% response rate).
DR-TB knowledge
The mean knowledge score was 4.7 points (95% confidence interval [CI]: 4.42–5.06), out of a possible maximum of 8 points. Response data are summarized in Table 1. The majority (85.1%) of respondents were able to correctly define multidrug-resistant (MDR) TB; however, less than a quarter (23.1%) knew the appropriate initial method for its diagnostic confirmation.
Students rated their knowledge about DS-TB higher than their knowledge about DR-TB. Most participants reported their knowledge of DS-TB to be either ‘excellent’ (20.7%) or ‘good’ (50.6%), whereas the knowledge of DR-TB was generally reported as ‘average’ (49.4%) or ‘below average’ (34.5%). The distribution of DR-TB knowledge scores was similar across the self-rated DR-TB knowledge categories (Fig. 1).
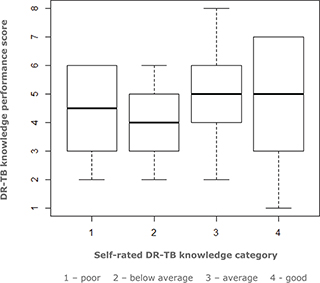
Fig. 1. Relationship between self-rated DR-TB knowledge and performance in DR-TB knowledge items.
Respondents identified factors relating to treatment non-adherence, comorbid immunosuppression, community transmission, and risk of TB exposure to be major contributors to the prevalence of DR-TB in South Africa (Fig. 2).
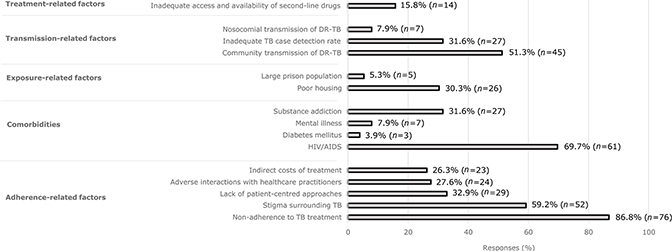
Fig. 2. Perceived factors contributing to DR-TB prevalence.
Perceived risk
Students were asked about their perceptions of personal risk of TB infection (Fig. 3). Three students reported a previous confirmed personal TB diagnosis. Most participants (85.1%) reported contact with a DR-TB patient in the past year. Over half (59.8%) also reported avoiding interacting with a patient because of a possible or confirmed DR-TB diagnosis. Students’ awareness of personal risk was also reflected in reported use of PPE. Most respondents (73.5%) claimed to frequently use an N95 respirator when sharing an environment with a patient with proven or possible TB. However, nearly half (47.7%) reported difficulties in finding an N95 respirator when needed.
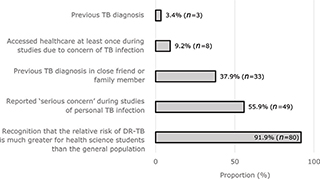
Fig. 3. Estimated risk and reported experiences of personal TB infection.
Institutional support
Respondents were asked about institutional support for students who develop occupational TB during their studies. Most participants believed that the primary responsibility for costs associated with occupational TB in medical students should fall to government (67.4%), rather than their academic institution (29.1%) or affected individuals (3.5%). More than half of the students (55.8%) were unaware of an institutional policy regarding occupational TB in students, while slightly more than a third (36.0%) of the students reported the current policy to be unacceptable. Specific measures that the vast majority of respondents believed should be provided in an institutional policy for support of TB-affected students were psychological support (94.3%), assistance with treatment costs (94.3%), and assistance with diagnostic costs (92%). Most participants (82.8%) had access to medical insurance, of whom 59.7% had comprehensive cover. There was no association between reported TB-related health-seeking behaviour and access to medical insurance (χ 2 = 1.84, P = 0.398).
Discussion
Due to the high prevalence of DR-TB in South Africa, undifferentiated clinical presentation between DR- and DS-TB, and progressive decentralization of DR-TB care, HCWs in all healthcare settings need to be aware of DR-TB diagnostics and principles of management. Junior clinicians should be equipped with the adequate knowledge and competencies to diagnose, treat, and prevent DR-TB at a primary care level. This should form part of the preparation medical students receive prior to starting clinical practice. In our study, medical students achieved a 59.2% average DR-TB knowledge score, which is similar to previously reported DS-TB knowledge in South African medical students (34).
Malangu et al. confirmed similar DR-TB knowledge gaps in HCWs in Lesotho (20). These deficits in TB knowledge may contribute to discordance between prescribing practices and treatment guidelines (7), leading to ongoing TB transmission. Current undergraduate training in DR-TB care appears to be inadequate in high TB burden settings, such as South Africa. This training should be better incorporated into undergraduate medical curricula to prepare junior clinicians for the roles they are required to play in DR-TB prevention and control. Targeted DR-TB training should incorporate key basic science and clinical principles, which may be addressed through lectures and tutorials. Additionally, medical students should be instructed in relevant clinical skills and infection control strategies, which may be imparted through simulation training and role-modeling by senior clinicians in clinical learning environments. Addressing deficits in DR-TB knowledge should be the responsibility of institutions of medical education.
Shah et al. estimated that 70% of DR-TB cases arise due to primary transmission of a resistant strain, rather than via de novo resistance associated with previous treatment exposure (35–39). In our study, ‘non-adherence to TB treatment’ was the most frequently identified item in a selection of 15 possible factors contributing to the DR-TB epidemic. This misperception, also held historically by many senior colleagues, may lead clinicians to hold an unfair bias against DR-TB patients and may lead them to underestimate the risk of drug resistance in patients without a history of previous TB (9, 10). Human immunodeficiency virus (HIV) infection/acquired immune deficiency syndrome (AIDS) has been identified as an important risk factor for DR-TB (32, 40) and was identified as such by a majority of respondents.
Training on the risk of occupational diseases and risk mitigation strategies should form part of standard undergraduate medical education. Medical students should also be protected against occupational diseases during their studies. In our study, participants demonstrated a high level of awareness regarding personal risk of occupational TB, with 92% correctly considering their risk to be higher than that of the general population. A similar risk perception was found among medical students at another South African university 5 years prior (34). This contrasts with the reports of the senior South African HCWs underestimating their personal risk (19, 41). This may represent a change whereby junior HCWs acknowledge their risk of occupational TB (19, 25) as part of a broader shift in which self-care is progressively valued more highly by junior HCWs (42, 43). This cohort was exposed to sensitizing ideas regarding personal TB risk during engagement with the advocacy group TB Proof (44), which promotes awareness and prevention of occupational TB, and helped to establish undergraduate TB IPC training and N95 respirator fit testing at this university. Individuals within the class who had been treated for TB have previously shared their stories and, in one prominent example, also shared information about their experience on public platforms to raise awareness and combat TB-related stigma (45). This suggests that this student cohort’s understanding of TB has been shaped by a combination of personal and clinical experiences, correlating with previous reports that sharing stories of HCWs with occupational TB can drive behavioral change (16, 19).
Participants expressed a strong intention to use PPE, but reported problems with availability. Our finding that approximately half of the students experienced challenges in accessing N95 respirator masks is similar to the findings in another large academic hospital in South Africa (34), even though Department of Health policy dictates that these should be readily available at all times. These challenges have been greatly intensified by dire local and global shortages of N95 respirators and other PPE related to the COVID-19 pandemic. In TB-endemic areas, PPE shortages expose HCWs to a dual risk of SARS-CoV-2 and TB infection (46). The National Department of Health should prioritize and strengthen PPE supply chains, and institutions of medical education should ensure that students have consistent access to essential PPE within clinical environments.
TB and, more recently, COVID-19 are sources of anxiety and real danger for medical students undergoing training in clinical settings. Yet unlike their ‘employed’ counterparts, students in South Africa are currently not protected by the Compensation for Occupational Injuries and Diseases Act, should they develop and suffer consequences due to an occupational illness (47). In our study, medical students reported significant DR-TB exposure (85% reported contact with DR-TB patients). This is consistent with high rates of occupational DR-TB described in medical students in South Africa and India (14, 18). Despite this risk, routine screening of healthcare students and staff for TB has not been implemented widely in South Africa. Although many students reported a concern of personal TB infection, few (9.2%) had sought healthcare to obtain a diagnosis and treatment. This could be due to significant barriers to TB diagnosis and care for medical students, including academic obligations, stigma, and indirect costs, such as transport expenses (18, 19). We did not find an association between TB-related health seeking behaviour and private medical insurance, but the number of uninsured participants was small (17% vs. a national average of 84–88% among this age group) (48), possibly representing a source of sampling bias. Although students have access to free TB work-up and treatment in the public sector, they may be discouraged from seeking care here by long waiting times, fear of encountering fellow students or practitioners, and a lack of confidentiality as their laboratory results and radiological studies would be accessible to fellow students and practitioners via centralized reporting systems. By contrast, medically insured students may choose to access care in the private sector but will be required to pay uninsured expenses out of pocket, as many medical insurance packages do not cover expenses for outpatient consultations and investigations. These dynamics highlight South Africa’s need for a national policy guiding occupational HIV and TB care for HCWs, including students (19, 41, 49). There has been limited progress to this end, with the National Occupational HIV/AIDS and TB Policy for Healthcare Workers in respect of HIV/AIDS and TB languishing in draft format since 2016. Health science students and professionals, with the support of their institutions, should advocate for better protection of health science students, which ultimately requires the release of this inclusive national policy followed by monitoring of its implementation.
Beyond curbing TB transmission, undergraduate IPC training contributes to the prevention of other infectious illnesses. The COVID-19 pandemic has drawn global attention to the importance of infection control and occupational health. The pandemic represents a unique opportunity to build more resilient health systems, in which all HCWs are protected and well prepared to face both ancient and novel pathogens.
This study was limited by a low response rate, potentially introducing a degree of participation bias. This may reflect a lack of interest in the subject or a lack of perceived personal relevance, or competing priorities prior to completing final medical examinations. The high rate of access to medical insurance observed in this sample suggests a high average socioeconomic status, potentially reflecting selection bias and limiting the generalizability of our findings. No validated assessment tool exists for this subject, which led to the design of a new questionnaire. This limits comparison with existing literature. The study was strengthened by a sample of individuals at the same point at the end of an undergraduate medical training program, prior to them working at different healthcare facilities across South Africa.
Conclusions
This study highlights the need to train medical students on the diagnosis, management, and prevention of DR-TB in high TB burden countries. The findings also highlight the importance of resilient PPE supply chains, IPC training, and comprehensive occupational health support, which have relevance to both DR-TB and novel pathogens, including SARS-CoV-2.
Effective strategies to attenuate the risk of occupational illnesses are urgently needed, starting with a comprehensive national occupational health policy that includes students. Medical students should be better prepared and protected as they face the complex challenges of novel and ancient pathogens in the era of antimicrobial resistance.
Declarations
Ethical approval and consent to participate
This study received ethical approval from the Human Research Ethics Committee of the University of Cape Town (HREC 582/2018). Written informed consent was obtained from each participant prior to enrolment into the study.
Consent for publication
The manuscript does not include any individual person’s data in any form.
Availability of data and materials
The consent form and questionnaire is available as Supplementary Material S1. The deidentified data set that support the conclusions of this study is available as Supplementary Material S2.
Acknowledgements
We would like to thank the UCT MBChB Class of 2018 for their participation and cooperation in this research. We would also like to thank Nicholas Loxton, Jared Tumiel, Bobo Mthombeni, Lauren Murray, Peace Francis, Micayla Pather, Caleb Langton, and Rachel Gardiner for their participation and feedback in the pilot study. Finally, we would like to acknowledge the UCT School of Public Health and Family Medicine for its support.
Authors’ contributions
All authors (MH, JW, MJR, AvD, and HMW) contributed to the conception and design of the study. The data were collected by MH, JW, and MJR. Data analysis was performed by MJR, with input from JW and MH. All authors interpreted the data. All authors drafted or substantively revised this manuscript and approved the final manuscript prior to submission.