ORIGINAL ARTICLE
Characterization of an outbreak due to extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit
Paola Pidal1*, Erna Cona1, Carolina Torrejón1, Constanza Airola1, Javier Cifuentes2, Sergio Ambiado2, Annette Navarrete2, Eliana Chacon1, Iris Valderrama1, Velimir Mihalic3, Fabian Aravena3, David Gómez3, Ingrid Araya4 and Loredana Arata5
1Healthcare-Associated Infections Service, Clinica Indisa, Santiago, Chile; 2Neonatal Critical Patient Service, Clinica Indisa, Santiago, Chile; 3Microbiology Laboratory, Bionet, Santiago, Chile; 4Bacteriology Section, Institute of Public Health of Chile, Santiago, Chile; 5Molecular Genetics Sub Department, Institute of Public Health of Chile, Santiago, Chile
Abstract
Background: The epidemiological–microbiological characteristics and effective intervention measures in an outbreak due to extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae in a neonatal intensive care unit are described.
Materials and methods: Cases from June 22, 2018 to February 11, 2019 were analyzed. Microbiological analysis of intestinal carriage of ESBL-producing K. pneumoniae and environmental samples was conducted. Strain relationships were studied by pulsed-field gel electrophoresis (PFGE).
Results: A 35-week outbreak affecting 41 patients, with eight infected, 33 colonized, and two deceased patients occurred. Two stages of the outbreak were observed with differences in the frequency of intestinal carriage of ESBL-producing K. pneumoniae. The same genetic subtype was seen in patient strains and was different from strains isolated from the environment. Deficiencies in contact precautions, hand hygiene, and handling of breast milk were observed.
Conclusions: A monoclonal outbreak by ESBL-producing K. pneumoniae that occurred in two phases and the different control measures in each of the stages is described. Effective control measures are mainly based on improving compliance with standard precautions and contact precautions, and other complementary measures are described such as proper handling of breast milk, periodic carriage studies, and the generation of three patient cohorts.
Keywords: health care-associated infections; outbreaks; klebsiella pneumoniae; antibiotic resistance; extended-spectrum β-lactamase; neonatal intensive care; Chile
Citation: Int J Infect Control 2021, 17: 20916 – http://dx.doi.org/10.3396/ijic.v17.20916
Copyright: © 2021 Paola Pidal et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 9 October 2020; Revised: 8 July 2021; Accepted: 11 July 2021; Published: 02 September 2021
Competing interests and funding: The authors declare they have no potential conflicts of interest.
*Paola Pidal, Clinica Indisa, Sta. María 1810, Providencia, Santiago, Región Metropolitana, PO Box 7520440, Chile. Email: paola.pidal@indisa.cl
Health care-associated infections (HAIs) pose a challenge in neonatal critical care units and are a major cause of morbidity and mortality and of rising care costs (1). The breakthroughs in science and technology over the past decades have made it possible to significantly improve survival of young gestational age and very low birth weight newborns, both being major risk factors of getting HAI (2, 3).
Klebsiella pneumoniae is considered a major etiological agent of HAI and the main gram-negative bacilli associated with nosocomial outbreaks in neonatal intensive care units, the newborn being particularly vulnerable to colonization and infection with this agent (4). It is normally present in the gastrointestinal tract of neonates, which is its main reservoir, and is also capable of surviving on environmental surfaces, thus creating multiple opportunities for transmission in a hospital context (5, 6). K. pneumoniae has various resistance mechanisms, including the extended-spectrum β-lactamases (ESBLs), enzymes capable of hydrolyzing broad-spectrum cephalosporins, and monobactams. The global spread of ESBL-producing strains of K. pneumoniae is a serious threat to public health; in hospitals, the proportion is estimated to exceed 30% in most of the world and more than 60% in some regions of the world (7, 8). In Chile, the HAI national report showed a 34.7% susceptibility to third-generation cephalosporins in 1,148 strains of K. pneumoniae in 2018 (9). Stapleton et al. in a review of outbreaks due to ESBL-producing Enterobacteriaceae in neonatal intensive care units describe a high fatality rate in infected newborns, reaching 31% (95% CI: 20–43%). In their review, ESBL-producing K. pneumoniae was responsible for 69% of the outbreaks analyzed (9). The intervention measures described to control these outbreaks included those aimed at strengthening hand hygiene, screening of the environment, health care team, and patients, as well as the generation of a patient cohort and improved environmental hygiene; measures such as unit closure and cohorting of staff were described less frequently (10).
Prominent among the transmission mechanisms described in the various outbreaks due to ESBL-producing K. pneumoniae in neonatal intensive care units is person-to-person transmission, although there are reports of transmission related to contamination of intravenous solutions and breast milk (11–16). ESBL-producing K. pneumoniae has also been isolated from environmental sources such as soap solutions; multiple studies have described that sinks and drainage systems can turn into reservoirs of multi-resistant gram-negative bacteria, and they have been associated as possible sources of infection. However, it is difficult to establish a causal relationship (17–19). The stability of some plasmids in certain ESBL-producing K. pneumoniae lineages suggests the existence of various genetic elements that may contribute to invasion as well as to the persistence of certain strains in the hospital environment; therefore, it is theoretically possible to consider an environmental source (20–21).
This study aims to describe the epidemiological and microbiological characteristics as well as the intervention measures in the control of an ESBL-producing K. pneumoniae outbreak in a neonatal critical patient unit (CPU).
Methods
An observational descriptive study with intervention measures was conducted covering the period from the detection of the primary case on June 22, 2018 to February 11, 2019, the date when the last ESBL-producing K. pneumoniae isolate was detected. This study was conducted in a high-complexity private hospital in Santiago, Chile, which has 400 beds distributed in clinical, surgical, and intensive care areas. The neonatal CPU has 16 intensive care (ICU) spaces and 26 intermediate care (IMCU) spaces, providing care for neonatal patients with high-risk pathologies, including preterm and full-term newborns with serious illnesses and/or severe congenital malformations, requiring extended hospitalization, use of invasive medical and surgical procedures, and life support; 30% of admissions in 2018 were newborns <32 weeks and/or <1,500 g birth weight.
Case detection was carried out through the active epidemiological surveillance system based on clinical and laboratory surveillances for HAIs. The HAI control team includes trained nurses, a medical doctor specialized in HAI and hospital epidemiology, and a microbiologist. The diagnosis of HAI was made according to the definitions of the national infection control program (9). Demographic data for each patient, as well as invasive procedures, were recorded in standardized documents. During the outbreak period, structured and unstructured supervisions of the basic HAI prevention measures were carried out, including technique and opportunity of hand hygiene, contact precautions, cleaning/disinfection, breast milk extraction in the neonatal unit, and milk fortification in the milk preparation unit.
The neonatal unit has standardized protocols for the use of antimicrobials and surgical prophylaxis: ampicillin and gentamicin in early sepsis and vancomycin with amikacin in late sepsis, changing the antimicrobials in accordance with the results of cultures. An antimicrobial stewardship program has not been implemented in the unit and was not implemented during the outbreak.
During the outbreak period, 398 inguino-rectal samples were obtained for the study of ESBL-producing K. pneumoniae. At week 12 of the outbreak, samples were obtained from patients with specific risk factors (mechanical ventilation and/or gastrectomy), and at week 26, they were obtained from all hospitalized patients. Subsequently, screening was continued weekly until week 35 (considered the end date of the outbreak), then every 15 days for 2 months, and finally monthly for an additional 2 months. Clinical samples for diagnostic-therapeutic purposes were taken at the clinical unit and processed in the local microbiology laboratory following the standard procedure according to the infectious focus. Inguinal/rectal smear samples were taken to study the carriage of ESBL-producing K. pneumoniae, which were subsequently inoculated in the laboratory on CHROMID® ESBL screening agar medium (BioMerieux, Marcy-l’Étoile, France). Bacterial identification was carried out by the MALDI TOF (matrix-assisted laser desorption ionization time-of-flight) system VITEK® MS (BioMerieux, Marcy-l’Étoile, France); the minimum inhibitory concentration (MIC) to antimicrobials was determined through the automated Phoenix® system (Becton Dickinson, Franklin Lakes, NJ, USA), and ESBL detection was carried out by the disk diffusion method described by the Clinical Laboratory Standards Institute (CLSI) (22). Current CLSI criteria were used to interpret the results.
Fifty environmental samples were obtained and selected according to the following criteria: two samples from the surface of the transport crib; 14 ammonium bottles that were transferred from a central quaternary ammonium drum; one hand cream in use that was shared to health care workers to moisturize their hands; four samples of chlorhexidine soap located in patient rooms (two from colonized and two from non-colonized patients); seven samples of alcohol gel in use located in patient rooms (four colonized and three non-colonized patients); 10 samples from sink drains (from five colonized and five non-colonized patients); and 12 sink traps (six from colonized and six from non-colonized patient sinks). Fourteen samples of breast milk randomly selected from 10 mothers with colonized children and two mothers with non-colonized children were included. The environmental samples were specifically targeted to the search for a reservoir of ESBL-producing K. pneumoniae. For the collection of liquid samples, 1–5 mL of each solution was introduced into sterile tubes, and for the dry surface samples, a sterile swab previously moistened in sterile saline serum and subsequently introduced into Stuart T’enT’-SS® transport medium (Winkler, Santiago, Chile) was used. All samples were immediately taken to the microbiology laboratory. In the laboratory, the samples were inoculated in brain heart broth and incubated at 35 ± 2°C for 48 h, and then they were inoculated on selective CHROMID® ESBL agar screening medium. Post-fortification breast milk samples were also obtained, and 1–5 mL was introduced into sterile tubes; later in the laboratory, they were inoculated into brain heart broth and incubated at 35 ± 2°C for 48 h, and then they were inoculated onto blood agar medium (BioMerieux, Marcy-l’Étoile, France).
A group of ESBL-producing strains of K. pneumoniae from clinical and environmental samples was sent to the reference center (Institute of Public Health of Chile) for confirmation and analysis of clonal relationship by means of molecular study by pulsed-field gel electrophoresis (PFGE). Agarose templates were prepared for PFGE according to the standardized protocol of the PulseNet Network (Centers for Disease Control and Prevention) (http://www.cdc.gov/pulsenet/pathogens/index.html). The strains were incubated in brain heart broth, and subsequently, these cultures were soaked in Sea Kem Gold 1% agarose (Lonza, Basel, Switzerland), being digested for 4 h at 37°C with the SpeI enzyme (30U/template; Thermo Scientific, Waltham, MA, USA). The Salmonella serotype Braenderup strain H9812 (ATCC BAA 664) was used as the size standard. The gels were stained with Gel Red (Biotium, Fremont, CA, USA) and visualized on a Gel Doc XR equipment (BioRad, Hercules, CA, USA). Data analysis was performed using BioNumerics v6.6 (Applied Maths NV, https://www.applied-maths.com). For SpeI, the different patterns were grouped into pulsotypes based on a cut-off value of 95% pattern similarity. Pattern grouping analysis was performed using the unweighted pair group method (UPGMA).
This study was evaluated by the scientific ethics committee of the Eastern Metropolitan Health Service, in accordance with Law 20120 and in line with the recommendations of the Ministerial Commission for Health Research, obtaining the corresponding approvals.
Results
Figure 1 shows the number of isolates of ESBL-producing K. pneumoniae during the 35 weeks of the outbreak, from June 22, 2018, when the primary case was detected, to February 11, 2019, when the last strain (which came from a urine sample from an infected patient) was isolated. After this date, no new cases of patients infected by this agent were identified. In the pre-epidemic period from January 2017 to June 21, 2018, no ESBL-producing strains of K. pneumoniae were isolated in neonatal CPU patients.
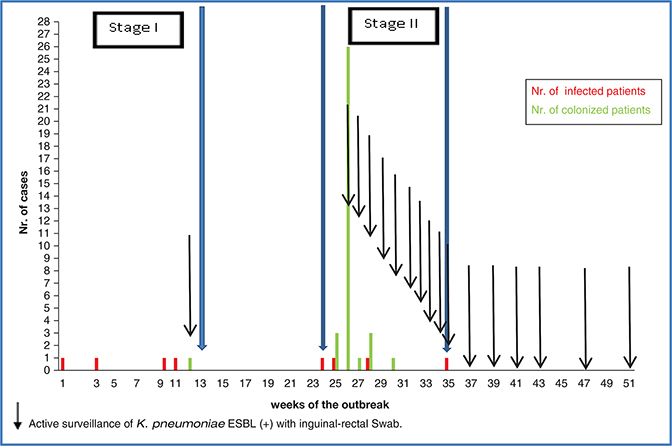
Fig. 1. Time curve of events during the outbreak due to ESBL-producing K. pneumoniae in the neonatal intensive care unit.
During the outbreak period, 306 newborns remained hospitalized in neonatal CPU – 86 of them were very preterm infants (<32 weeks). The length of stay of the newborns was 17.4 + 24.7 days (average + SD). During the outbreak period, the median census of the NICU was 37 newborns (interquartile range: 33–39). ESBL-producing K. pneumoniae was isolated in 41 (13.4%) from either a clinical sample taken for diagnostic purposes or inguinal-rectal swab as part of active surveillance; eight cases (2.6%) corresponded to infected patients and 33 (10.8%) to patients who were only colonized with this agent. When analyzing the number of new cases per week of appearance, a total of 43 cases is seen because two patients were first colonized and later developed an infection by the same agent (Fig. 1).
In the 41 patients who were part of the outbreak, the average birth weight was 1,496 g, with an average gestational age of 30 weeks and average APGAR at 1 min of 7 (Table 1).
The outbreak had two epidemiologically well-differentiated periods, considering day 1 as the date of detection of the primary case: the first period ranged from week 1 to week 12, and the second period ranged from week 24 to week 35, divided by a 12-week period in which no new cases of infection were detected.
Description of the first period
Four infected patients and one colonized patient were detected and distributed throughout the period, accounting for 2.8 and 0.7% of hospitalized patients in this period. The colonized patient was detected in the active surveillance of inguinal-rectal swabs performed at week 12. Of the four cases of infection, two patients had bacteremia, one patient had urinary tract infection not associated with an indwelling urinary catheter, and one patient had ventilator-associated tracheobronchitis. A newborn – a 26-week premature infant with a 570 g of birth weight – who had signs and symptoms of sepsis died. Outbreak control measures at this stage included contact precautions for colonized and infected patients, unstructured monitoring of HAI control practices, and active search for carriers of ESBL-producing K. pneumoniae. Surveillance was performed only at week 12 and included nine high-risk patients (mechanical ventilation and/or gastrostomy) of the 38 hospitalized patients, one of whom (11%) was positive for ESBL-producing K. pneumoniae. In the unstructured supervision of the practices, deficiencies in contact precautions and hand hygiene were observed.
Between weeks 13 and 23 of the outbreak, no new infections with ESBL-producing K. pneumoniae were described. At week 21, the last patient in whom ESBL-producing K. pneumoniae was isolated in the previous period was discharged, remaining with permanent contact precautions. In this stage, a training program on standard precautions and contact precautions was carried out by the HAI control team. Active surveillance cultures were not performed.
Description of the second period
Between weeks 24 and 35 of the outbreak, four infected patients and 32 patients colonized with ESBL-producing K. pneumoniae were identified, accounting for 4.3 and 34% of hospitalized patients, respectively. This second stage of the outbreak became apparent based on two infected cases (Fig. 1), after which all patients in the unit were evaluated. Infections included one case each of sepsis with an abdominal focus, central venous catheter-associated bacteremia, urinary tract infection not associated with an indwelling urinary catheter, and conjunctivitis. One patient, a premature newborn of 25 weeks of gestation and 840 g birth weight, died of sepsis secondary to intestinal perforation in the context of necrotizing enterocolitis. Of the 41 patients hospitalized at week 26 of the outbreak, 34 (83%) underwent active surveillance through inguinal-rectal swab, 26 of whom (76%) were positive for ESBL-producing K. pneumoniae. In the supervision of practices, deficiencies related to the process of breast milk extraction stood out, particularly with clean area management and the mothers’ hand hygiene, as well as in the breast milk fortification area where deficiencies were seen in the identification of bottles and washing of utensils used to fortify breast milk. Hand hygiene compliance ranged between 82 and 90%, contact precautions between 63 and 100%, and hand hygiene technique between 29 and 100%; deficiencies related to the duration of hand hygiene were detected. From a structural point of view, some IMCUs did not have sinks inside, making access to hand washing difficult, and sinks with pipes connected in parallel between adjoining patient rooms were also observed. No equipment or supplies that were shared between patients were detected. The outbreak control measures in this second stage and added to those previously implemented included the following: structured supervision of practices such as timing and technique of hand hygiene; contact precautions; weekly active surveillance of inguinal-rectal swabs in search of ESBL-producing K. pneumoniae from week 26 to week 35, which marked the end of the outbreak; and a search for an environmental reservoir that could explain a common source of exposure. Three cohorts for patient care were created: a cohort for new admissions, in a separate physical facility and reconditioned for these purposes; the second cohort of patients hospitalized in the neonatology CPU during the period of the outbreak, but with negative surveillance cultures; and a third cohort of patients with isolation of ESBL-producing K. pneumoniae in any sample; each cohort had separated nursing staff. Supervision was strengthened in the unit with a professional exclusively dedicated to this activity, and preventive measures for the spread of nosocomial agents were mainly reinforced throughout the breast milk extraction, collection, and fortification processes, both in the clinical service and in the milk preparation service. The disinfectant used on high-contact surfaces of the patient unit was changed from quaternary ammonium sprays to hydrogen peroxide disposable towels, as well as the use of a bioluminometer to monitor the quality of environmental hygiene at discharge in all the rooms of discharged patients colonized or infected with ESBL-producing K. pneumoniae. All deficiencies previously described were corrected during this second phase of the outbreak, and structural improvements are also made in the neonatology CPU, including the implementation of new sink systems inside the units, and a change in the sink drainage system to avoid parallel connection between sinks in adjoining patient rooms.
Post-outbreak follow-up
Active surveillance for carriage of ESBL-producing K. pneumoniae was continued for a period of 4 months since the end of the outbreak. All patients who remained hospitalized on the day of the study were included. Surveillance was carried out every 15 days in the first 2 months and once a month during the last 2 months. No new cases were identified during this time. A program of continuous supervision of opportunity and hand hygiene technique was established as well as contact precautions, observing compliance over 90% in each of them during 2019.
Microbiology
Thirty-eight (93%) of the 41 ESBL-producing strains of K. pneumoniae isolated from the patients were sent to the National Reference Center, Public Health Institute of Chile. The 38 strains had identical phenotypic resistance characteristics: in 100%, the existence of ESBL was demonstrated, and the presence of carbapenemases was ruled out. All isolates were also resistant to ciprofloxacin and gentamicin. In seven strains of ESBL-producing K. pneumoniae isolated from clinical samples, the molecular study of resistance genes demonstrated the carriage of blaCTX-M, blaTEM, and blaSHV. Of the 50 environmental cultures performed, eight were ESBL-producing K. pneumoniae: three isolated from sink traps in three rooms of the ICU, four from the IMCU corresponding to traps of sinks located outside the patient room and shared for two rooms, and one from an ICU room sink drain. It is worth noting that of the 12 cultures performed on sink traps, seven (58%) were positive for ESBL-producing K. pneumoniae. The remaining cultures including fortified breast milk were negative for ESBL-producing K. pneumoniae. In 100% of the isolated environmental strains, the existence of ESBL was demonstrated, and the presence of carbapenemases was ruled out; in addition, two groups differentiated mainly by their sensitivity to gentamicin and ciprofloxacin were seen, which were also genetically differentiated.
Clonality analysis
Forty-six ESBL-producing strains of K. pneumoniae (38 clinical samples and eight environmental samples) were sent to the National Reference Center for confirmation and molecular typing study. Thirty-seven of the 38 strains isolated from clinical samples showed the same pattern of bands in the PFGE analysis, corresponding to the same genetic subtype called clone 221, and one strain showed another pattern of bands (clone 225), but it was closely related to clone 221 with 94% similarity. The eight strains isolated from the environment had six different genetic subtypes, which were grouped into two large genetically well-differentiated groups with a similarity of only 75% between them, and with a different band pattern from the clinical isolates with a similarity of only 85 and 75%, respectively, with clone 221 (Fig. 2).
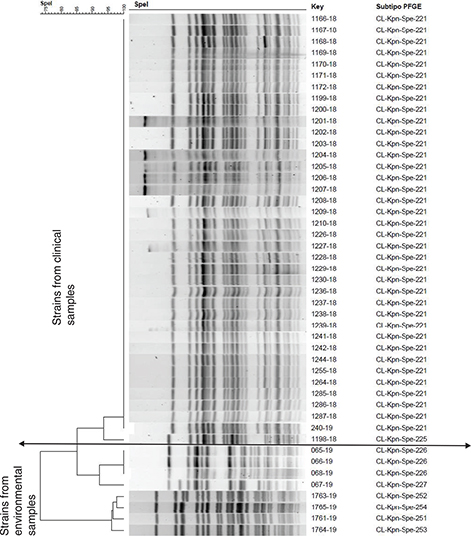
Fig. 2. Molecular analysis of 46 strains of ESBL-producing K. pneumoniae by pulsed-field gel electrophoresis.
Discussion
A 35-week ESBL-producing K. pneumoniae outbreak is described in a neonatal CPU, affecting 41 patients (eight infected and 33 colonized, accounting for 2.6 and 10.8% of hospitalized patients in the period, respectively). Two infected patients (25%) died of the infection, both extremely premature infants.
The outbreak had two epidemiologically well-differentiated periods, mainly due to the colonization frequency, reaching 0.7 and 34% between the first and second stages, respectively. While in the first stage of the outbreak, active surveillance of carriage was carried out only at the end of the period (week 12 of the outbreak), it focused on those considered at risk, and only one carrier of ESBL-producing K. pneumoniae was detected, accounting for 11% of patients monitored. In contrast, the second study carried out in week 26 of the outbreak corresponded to the second stage, where 76% (n = 26) of those tested were positive for ESBL-producing K. pneumoniae.
There are limitations in our study, mainly related to the performance of active surveillance culture in the first phase of the outbreak (week 12) performed only in patients with mechanical ventilation and/or gastrostomy, and the lack of active surveillance between weeks 13 and 26. This does not allow to rule out that our outbreak was prolonged, instead of having two different phases. Another important limitation is the absence of an assessment of risk factors for infection or colonization by ESBL-producing K. pneumoniae.
Despite the limitations stated, the difference in the prevalence of fecal carriage of the agent between the two analyzes leads us to set out different causal hypotheses for the spread of the agent in each period. In the first stage of the outbreak and based on the deficiencies identified in compliance with hand hygiene technique and contact precautions, we propose cross-transmission via hand carriage from reservoir patients as a probable cause for the outbreak. However, in the second stage of the outbreak, the accumulation of many cases in a short period of time makes it possible to suggest that an exposure to a common source could have occurred after week 13, and that it may explain the high prevalence of intestinal colonized patients in the week 26 of the outbreak. We were unable to identify a common source in the second stage of the outbreak, but we did not completely rule out this possibility. Due to the deficiencies observed in the identification of bottles and washing of utensils used to fortify breast milk, a possible hypothesis could be the contamination of breast milk in the extraction period by mothers of newborns colonized with this agent and a possible subsequent contamination of other maternal milk in the fortification process, although we were unable to prove this. There are reports that have related outbreaks in neonatology with contaminated breast milk (14, 15, 23, 24), so we recommend considering this possible source in the analysis of outbreaks in neonatal units, in addition to incorporating it as an essential process within the HAI prevention program.
Within the outbreak control measures and given the high prevalence of ESBL-producing K. pneumoniae carriage found in week 26 of the outbreak, it was decided to open three completely physically differentiated patient care cohorts with exclusive nursing staff to each of them; one of these cohorts was exclusively for new patients who entered the unit. We considered this key strategy to successfully control the outbreak in a short period of time from its implementation in the second stage, as well as weekly follow-up with a study of intestinal carriage of ESBL-producing K. pneumoniae to monitor the effectiveness of prevention measures until a complete control was achieved.
Multiple studies have described that sinks and drainage systems can be contaminated and be reservoirs of multi-resistant gram-negative bacteria (18, 19). These reservoirs can be created by misuse of sinks when disposing waste with contaminated organic material and are favored by poor condition or design of drain pipes, with frequent horizontal sections or even with a negative slope and sometimes blocked sections, leading to stagnation and subsequent biofilm formation, which end up contaminating the sink and consequently the hands of the staff and their clothing when using it. Of the cultures performed on the traps, seven were positive for ESBL-producing K. pneumoniae. Despite the fact that the molecular study did not show a clonal relationship with the strains isolated from clinical samples, the sink system was modified for a deeper one with an automatic opening system, and the drainage system was changed to a copper pipe system without negative slopes and without parallel connections between sinks in adjoining rooms. In our particular case, the ESBL-producing strains of K. pneumoniae found were not related to the outbreak, but it is possible that in other cases, they are environmental reservoirs of multi-resistant agents that may be the cause of outbreak prolongation.
The main contribution of this study is to show the lessons learned from this outbreak, mainly in the management and study in the first stage. Among the lessons learned in the prevention and management of this outbreak, we consider it is essential to maintain a structured and systematic supervision of the basic practices of HAI, such as hand hygiene and specific precautions, the establishment of an active surveillance program for ESBL-producing strains of K. pneumoniae strains, and the maintenance of these for a period of time after the end of the outbreak. The latter would have allowed us to have a better follow-up of the cases after the first phase of the outbreak and to have acted in a more timely manner. It is also important to strengthen other risk practices such as the breast milk process. Other additional measures such as the generation of differentiated patient cohorts can be especially useful when there is a considerable number of cases; this allowed us in the second phase of the outbreak to control it as quickly as possible and not close the unit to new admissions. This is the first report in our country of isolation of ESBL-producing K. pneumoniae in sink traps in a neonatal unit, and despite not being related to the cause of this outbreak, it shows an environmental reservoir of multi-resistant bacteria that could be a source of HAI.
In conclusion, ESBL-producing K. pneumoniae is an agent causing outbreaks in neonatal units. Control measures should focus on compliance with standard and contact precautions, but it is also important to evaluate and strengthen other risk practices such as those associated with breast milk extraction and fortification. We also consider it useful to implement differentiated patient cohorts and active surveillance of intestinal carriage in this type of outbreak in order to control it as quickly as possible.
References
- Magill SS, O’Leary E, Janelle SJ, Dumyati G, Nadle J, Wilson LE. et al. Changes in prevalence of health care–associated infections in U.S. Hospitals. N Engl J Med 2018; 379(18): 1732–44. doi: 10.1056/NEJMoa1801550
- Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: A prospective population-based cohort study. J Pediatr 2018; 201: 106–14.e4. doi: 10.1016/j.jpeds.2018.05.048
- Li X, Xu X, Yang X, Luo M, Liu P, Su K et al. Risk factors for infection and/or colonization with extended-spectrum β-lactamase-producing bacteria in the neonatal intensive care unit: a meta-analysis. Int J Antimicrob Agents 2017; 50(5): 622–8. doi: 10.1016/j.ijantimicag.2017.06.027
- Johnson J, Quach C. Outbreaks in the neonatal ICU. Curr Opin Infect Dis 2017; 30(4): 395–403. doi: 10.1097/QCO.0000000000000383
- Das P, Singh AK, Pal T, Dasgupta S, Ramamurthy T, Basu S. Colonization of the gut with gram-negative bacilli, its association with neonatal sepsis and its clinical relevance in a developing country. J Med Microbiol. 2011; 60, 1651–1660. doi: 10.1099/jmm.0.033803-0
- Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 2016; 62: 1423–1435. doi: 10.1093/cid/ciw122
- Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983; 11(6): 315–7. doi: 10.1007/BF01641355
- Organización mundial de la salud. Antimicrobial resistance. Global report on surveillance. 2014. Ginebra [Suiza]: Prensa de la OMS; 2014
- Ministerio de salud. Informe de vigilancia de infecciones asociadas a la atención en salud. August 2018. Chile. Available from: http://web.minsal.cl/infeccionesintrahospitalarias
- Stapleton PJM, Murphy M, McCallion N, Brennan M, Cunney R, Drew RJ. Outbreaks of extended spectrum beta-lactamase producing Enterobacteriaceae in neonatal intensive care units: A systematic review. Arch Dis Child Fetal Neonatal Ed 2016; 101(1): F72–8. doi: 10.1136/archdischild-2015-308707
- Haller S, Eller C, Hermes J, Kaase M, Steglich M, Radonić A, et al. What caused the outbreak of ESBL-producing Klebsiella pneumoniae in a neonatal intensive care unit, Germany 2009 to 2012? Reconstructing transmission with epidemiological analysis and whole-genome sequencing. BMJ Open. 2015; 5(5): 1–9. doi: 10.1136/bmjopen-2014-007397
- Corbella M, Caltagirone M, Gaiarsa S, Mariani B, Sassera D, Bitar I, et al. Characterization of an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit in Italy. Microb Drug Resist. 2018;24(8):1128–36. doi: 10.1089/mdr.2017.0270
- Rettedal S, Löhr IH, Natås O, Giske CG, Sundsfjord A, Øymar K. First outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a Norwegian neonatal intensive care unit; associated with contaminated breast milk and resolved by strict cohorting. Apmis 2012; 120(8): 612–21. doi: 10.1111/j.1600-0463.2012.02879.x
- Leigh G. Donowitz, Frederic J. Marsik, Karen A. Fisher, Richard P. Wenzel, Contaminated breast milk: A source of Klebsiella bacteremia in a newborn intensive care unit. Reviews of Infectious Diseases, 1981; 3(4): 716–20. doi: 10.1093/clinids/3.4.716
- Dorota P, Chmielarczyk A, Katarzyna L, Piotr M, Lauterbach J, et al. Klebsiella pneumoniae in breast milk-a cause of sepsis in neonate. Arch Med 2017, 9: 1. doi: 10.21767/1989-5216.1000189
- Gray J, Arvelo W, McCracken J, Lopez B, Lessa FC, Kitchel B, et al. An outbreak of Klebsiella pneumoniae late-onset sepsis in a neonatal intensive care unit in Guatemala. Am J Infect Control 2012; 40(6): 516–20. doi: 10.1016/j.ajic.2012.02.031
- González RAC, Gil GF, Solórzano RM, Cruz GJ, Puig PJ, Suárez SM, et al. Brote por Klebsiella pneumoniae multiresistente y productora de β-lactamasa de espectro extendido en una unidad de alto riesgo neonatal. Rev chil infectol 2011; 28: 28–34. doi: 10.4067/S0716-10182011000100005.
- Aranega-Bou P, George RP, Verlander NQ, Paton S, Bennet A, Moore G, TRACE investigators group. Carbapenem-resistant Enterobacteriaceae dispersal from sinks is linked to drain position and drainage rates in laboratory model system. J Hosp Infect 2019; 102:63–69. doi: 10.1016/j.jhin.2018.12.007
- De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H et al. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control 2017; 6: 24. doi: 10.1186/s13756-017-0182-3
- Sahly H, Navon-Venezia S, Roesler L, Hay A, Carmeli Y, Podschun R, et al. Extended-spectrum β-lactamase production is associated with an increase in cell invasion and expression of fimbrial adhesins in Klebsiella pneumoniae. Antimicrob Agents Chemother 2008; 52(9): 3029–34. doi: 10.1128/AAC.00010-08
- Löhr IH, Hülter N, Bernhoff E, Johnsen PJ, Sundsfjord A, Naseer U. Persistence of a pKPN3-like CTX-M-15-encoding IncFIIK plasmid in a Klebsiella pneumonia ST17 host during two years of intestinal colonization. PLoS One 2015; 10(3): 1–16. doi: 10.1371/journal.pone.0116516
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019.
- de Oliveira Garcia D, Doi Y, Szabo D, Adams-Haduch JM, Vaz TM, Leite D, et al. Multiclonal Outbreak of Klebsiella pneumoniae Producing Extended-Spectrum-Lactamase CTX-M-2 and Novel Variant CTX-M-59 in a Neonatal Intensive Care Unit in Brazil. Antimicrob Agents Chemother 2008 May; 52(5): 1790–1793. doi: 10.1128/AAC.01440-07
- Calbo E, Garau J. The changing epidemiology of hospital outbreaks due to ESBL-producing Klebsiella pneumoniae: the CTX-M-15 type consolidation. Future Microbiol 2015; 10(6): 1063–75. doi: 10.2217/fmb.15.22