REVIEW ARTICLE
A review of the science and clinical use of alcohol-based hand rubs
Elaine Ah-Gi Lo1, Lawrence Siu-Chun Law2*, Kevin Tan3 and Balakrishnan Ashokka3
1Department of Pharmacy, National University Hospital, Singapore, Singapore; 2Department of Medicine, National University Hospital, Singapore, Singapore; 3Department of Anaesthesia, National University Hospital, Singapore, Singapore
Abstract
Alcohol has a longstanding history as an antiseptic, and the coronavirus disease 2019 (COVID-19) pandemic has sparked a renewed interest in its use as a hand sanitizer. Alcohol works by denaturing protein and rendering cell membranes permeable. It offers excellent germicidal effects against Gram-positive and Gram-negative bacteria, Mycobacterium tuberculosis, fungi, and lipid-containing viruses. However, it is less reliable against non-lipid containing viruses and is ineffective against bacterial and fungal spores. Alcohol-based hand rub (ABHR) usually contains 60–90% isopropanol or ethanol. Additives such as chlorhexidine to complement the action of alcohol and emollients to ameliorate the drying effect of alcohol are often included to improve the formulation of ABHR. In the clinical setting, ABHR provides biocidal activity against multidrug resistant bacteria such as methicillin-resistant Staphylococcus aureus as well as viruses like human coronavirus, severe acute respiratory syndrome coronavirus, and Middle East respiratory syndrome. Moreover, its use is associated with an improved compliance with hand hygiene, which has been shown to translate into better patient outcomes. However, there are cases of intoxications secondary to ingestion of ABHR or adulterated alcohol when resources are diverted away from the normal beverage production to meet the increased need for hand sanitizer during the COVID-19 pandemic. The risk of unintentional topical absorption and fire hazard among healthcare workers is low but should not be ignored. We proposed recommendations to mitigate the risk of ABHR ingestion and poisoning as well as that of fire hazard.
Keywords: SARS-CoV-2; alcohol; hand sanitizer; ethanol; isopropanol; n-propanol
Citation: Int J Infect Control 2022, 18: 20611 – http://dx.doi.org/10.3396/ijic.v18.20611
Copyright: © 2022 Elaine Ah-Gi Lo et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 19 July 2020; Revised: 27 September 2020; Accepted: 13 January 2021; Published: 24 January 2022
Competing interests and funding: None reported. The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
*Lawrence Siu-Chun Law, Department of Medicine, National University Hospital, Singapore, 5 Lower Kent Ridge Road, Singapore 119074. Email: sclaw@alumni.duke.edu
Alcohol-based hand rub (ABHR) has gained popularity as an effective means of hand hygiene in healthcare settings (1, 2). When used appropriately, the effectiveness of ABHR has been demonstrated against a plethora of infectious microorganisms, including Gram-positive and Gram negative bacteria, mycobacteria, fungi, and viruses (2–5) Moreover, ABHR is easy to use, requiring only a short duration of time to exert biocidal effects (6). Consequently, ABHR is associated with improved hand hygiene effectiveness (7) and compliance (8) with hand hygiene practices. With the interest in ABVH rekindled during the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), this article aims to revisit the science and clinical use of ABHR. We begin by discussing the pharmacological and pharmaceutical properties of ABHR. We subsequently describe the clinical benefits and the hazards associated with its use in the clinical settings.
Pharmacology
Alcohol works by denaturing protein and rendering cell membranes permeable. The presence of a certain amount of water speeds up the process. Most alcohol hand rub formulations offer excellent germicidal effects against Gram-positive and Gram-negative bacteria, Mycobacterium tuberculosis, fungi, and lipid-containing viruses, as well as multidrug resistant pathogens such as vancomycin-resistant Enterococcus (VRE) and methicillin-resistant Staphylococcus aureus (MRSA). Their activity against non-lipid containing viruses is variable, and they are ineffective against bacterial and fungal spores (9, 10) Alcohol does not possess residual ability to inhibit microorganisms, and hence, hand rub should be frequently reapplied to maintain effective germicidal activity (9)
The antibacterial and antifungal effects of alcohol increase with the molecular weight in the following order of potency: (methanol) < ethanol < isopropanol < n-propanol (9). Methanol has the weakest bactericidal action of the alcohols and is seldom used in healthcare due to its high toxicity (10). Isopropanol has been shown to be more bactericidal than ethanol for Escherichia coli and S. aureus but is not as effective against non-lipid-containing viruses such as rotavirus and adenovirus when compared to ethanol (10). The antiseptic activity of n-propanol is superior to that of isopropanol and ethanol (5, 11). In theory, butanol would be a great antiseptic, given its higher molecular weight. However, its banana-like odor and its low solubility in water render it less suitable as an antiseptic.
Formulation
The type of alcohol
ABHR usually contains isopropanol or ethanol in various concentrations and combinations. n-propanol is approved in Europe, but not in the United States (US), as an active ingredient for skin antisepsis (12). The biochemical properties of the three alcohols are compared in Table 1. Ethanol has a lower molecular weight and, hence, a lower boiling point and greater vapor pressure when compared to isopropanol and n-propanol (13). This property renders ethanol more drying to the skin compared to isopropanol and n-propanol. At the right concentrations, ethanol is commonly consumed as a recreational beverage, but isopropanol and propanol are relatively potent central nervous system suppressants and are not suitable for ingestion. Therefore, the US Food and Drug Administration (FDA) emphasized the importance of ethanol-based hand rub to be denatured to minimize the risk of unintentional ingestion (14). This could be performed by adding poisonous/bitter additives to limit human consumption in accordance with the formulas provided in the Alcohol and Tobacco Tax and Trade Bureau regulation for alcohol in hand sanitizers, for example, formulae 40A or 40B (with or without the tertiary butyl alcohol [tert-butyl]) and formula 3C (isopropyl alcohol) (14). All three alcohols may be produced by chemical synthesis or fermentation. Their antiseptic spectrum and potency are similar, and the specific differences are highlighted in Table 1. n-propanol is a potent antiseptic, but its safety profile is not as well established compared to isopropanol and ethanol. It is a relatively unpopular choice for ABHR formulations (5).
| Ethanol | Isopropanol | n-propanol | |
| Chemistry (13) | Two carbon atoms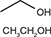 |
Three carbon atoms |
Three carbon atoms |
| Molecular weight: 46.1 | Molecular weight: 60.1 | Molecular weight: 60.1 | |
| Colorless | Colorless | Colorless | |
| Weak ethereal vinous odor | Odor of rubbing alcohol | Mild alcohol-like odor | |
| Miscible with water | Miscible with water | Miscible with water | |
| Boiling point: 173°F | Boiling point: 181°F | Boiling point: 207°F | |
| Vapor pressure: 44 mmHg | Vapor pressure: 33 mmHg | Vapor pressure: 15 mmHg | |
| Production | Hydration of ethene Fermentation by yeast |
Hydration of propene Fermentation by bacteria |
Hydrogenation of propionaldehyde Fractional distillation of fusel oil |
| Biology | Less toxic | More toxic, two to three times more potent CNS depressant | More toxic, two to three times more potent CNS depressant |
| More drying to skin | Less drying to skin | Less drying to skin | |
| Antiseptic spectrum/efficacy | More effective against non-lipid containing viruses such rotavirus and adenovirus (10) | More bactericidal than ethanol for E. coli and S. aureus (10) | Greater skin flora reduction compared to ethanol and isopropanol (11, 44) |
| CNS, central nervous system. | |||
Concentration
Concentrations of 60–90% are deemed the most effective, and 70% is typically chosen for hand rub preparations (9). While the antiseptic ability of alcohol drops sharply when its concentration falls below 60%, concentrations above 90% are not desirable for the following reasons: (1) pure alcohol dehydrates the cells and interferes with protein denaturation, rendering it less effective than a preparation with a lower alcohol concentration; (2) a higher concentration is more drying and irritating to the skin; (3) alcohol hand rub with a higher concentration evaporates faster.
Additives
Other than alcohol, additional antiseptics such as chlorhexidine and quaternary ammonium derivatives may be present in some formulations to complement the action of the principal component. For instance, adding chlorhexidine would help the preparation achieve a residual effect. Reichel et al. (11) also found chlorhexidine in conjunction with ethanol significantly enhanced log reduction of skin flora. Sporicidal agents such as hydrogen peroxides may be added to eliminate spores in the ingredients rather than for hand antisepsis. The use of hydrogen peroxide might, however, complicate the production owing to its corrosive nature and its difficult procurement in some countries (5). Emollients or humectants such as glycerol are often included in the formulations to reduce skin dryness and irritation. Water aids with antisepsis by speeding up protein denaturation by alcohol and is an essential ingredient in the production of hand rub. Colorants or fragrances are commonly used to increase the acceptability of the product but are associated with risk of allergic reactions and might increase the risk of ingestion by children. Dyes that leave stain or residue on the user’s hands or clothes should be avoided (15). Gelling agents and foaming agents may be added to help formulate the desired dosage form.
Examples
The World Health Organization (WHO) published a guide for local preparation to help healthcare facilities across different countries adopt ABHR as a standard of hand hygiene (5). The United States Pharmacopeia (USP) also released a document to provide compounding guidance for ABHR in view of sanitizer shortages associated with the COVID-19 pandemic (16). Table 2 lists the details of the WHO-recommended hand rub formulations as well as the USP alcohol-based sanitizer formulations with the formulations of two commercially available hand sanitizers. These formulations not only reflect the principles discussed above but also highlight the differences between local compounding and commercial manufacturing. All the listed formulations contain alcohol of 70–80% for optimal antiseptic effect and emollients to ameliorate the drying and irritating effect of alcohol to skin. Formulations for local compounding call for the addition of hydrogen peroxide as the process is associated with a higher risk of spore contamination. The commercially available formulations are usually more complicated and include fragrance and dye to increase the acceptability of the products. Chlorhexidine is added to one of the formulations to enhance the antiseptic effect of alcohol and gelling agents, such as crosspolymers are added to the other preparation to formulate the product into a gel. (17,18). Due to the flammable nature of alcohol and its potential to irritate mucosa (e.g. eyes, nasal, and gastrointestinal mucosa), ABHR is usually labeled to warn users of the potential risk.
| WHO Formulation 1 (44) USP Formulation 1 (16) |
WHO Formulation 2(44) USP Formulation 2 (16) |
USP Formulation 3 (16) | MICROSHIELD® Handrub Solution (17) |
PURELL® Healthcare Advanced Hand Sanitizer Gel (18) |
|
| Constituents | Ethanol 80% (v/v) Glycerol 1.45% (v/v) Hydrogen peroxide 0.125% (v/v) Water |
Isopropanol 79% (v/v) Glycerol 1.45% (v/v) Hydrogen peroxide 0.125% (v/v) Water |
Isopropanol 75% (v/v) Glycerol 1.45% (v/v) Hydrogen peroxide 0.125% (v/v) Water |
Active ingredients: Ethanol 70% (v/v) Chlorhexidine gluconate 0.5% (w/v) Inactive ingredients: Water Ethoxylated lanolin Glycerol Fragrance Dye |
Active ingredient: Ethanol 70% (v/v) Inactive ingredients: Water Isopropanol Caprylyl glycol Glycerin Isopropyl myristate Tocopheryl acetate Acrylates/C10-30 alkyl acrylate crosspolymer Aminomethyl propanol, fragrance |
| Warning labels | |||||
| Flammable | √ (WHO) | √ (WHO) | √ | √ | |
| External use only | √ (WHO, USP) | √ (WHO, USP) | √ (USP) | √ | √ |
| Keep out of reach of children | √ (WHO) | √ (WHO) | √ | ||
| Avoid contact with eyes | √ (WHO) | √ (WHO) | √ | √ | |
| Discontinue use if skin irritation or redness develops | √ | √ | |||
| Do not mix with detergents/other chemicals | √ | ||||
| USP, United States Pharmacopeia; WHO, World Health Organization; MICROSHIELD® (Schülke & Mayr GmbH, Nordersted, Germany); PURELL® (GOJO Industries, Inc., Akron, Ohio, USA). | |||||
Benefits of ABHR
The benefits of ABHR are broadly classified into effective biocidal activity and improved compliance with hand hygiene.
Effective biocidal activity
ABHR is effective against multiple infectious microorganisms as mentioned earlier. The bactericidal activity of isopropanol appears to be at least as effective as chlorhexidine and povidone-iodine. Isopropanol has been shown to reduce bacterial counts by 2.86 ± 1.22 log10 colony-forming units (CFUs), which is marginally superior to chlorhexidine and povidone iodine (19). Moreover, 70% isopropanol is comparable to 4% chlorhexidine at decreasing the load of normal flora on the hands of healthcare workers (20).
Interestingly, the introduction of ABHR in clinical environments is also associated with a reduction in nosocomial transmission of multidrug resistant bacteria such as MRSA and VRE (21) and extended-spectrum β-lactamase producing bacteria (22). This latter finding is particularly significant, given the increasing prevalence of multidrug resistant organisms in both acute (23) and chronic (24) healthcare settings globally.
In addition to bacteria, ABHR also demonstrates effectiveness against viruses. Indeed, hand hygiene with either 61.5% ethanol or 70% isopropanol effectively reduces in vitro viral loads after exposure to infectious doses of H1N1 influenza (3). Additionally, exposure of multiple Coronaviridae species to 70–95% ethanol or 50–100% isopropanol solutions for ≤1 min appears sufficient to inactivate human coronavirus, severe acute respiratory syndrome coronavirus, and Middle East respiratory syndrome coronavirus by a factor of ≥4 log10 in in vitro studies (25). In light of the latest COVID-19 pandemic, these findings are promising and suggest that SARS-CoV-2 might also be effectively inactivated by ABHR containing ethanol or isopropanol.
However, there are two major caveats to the use of ABHR. First, its activity against spores, for example, Clostridioides difficile, and non-enveloped viruses, for example, rotavirus and norovirus, is not reliable (9,10). Hand washing with soap and water is recommended when caring for patients with these pathogens. Soap and water is also recommended when hands are visibly soiled or dirty as the effectiveness of ABHR is limited in the presence of organic matter (5). Second, the key to the biocidal effectiveness of ABHR is the hand hygiene technique adopted by healthcare workers (26). The recommended procedure of hand rubbing with ABHR covers all areas of the hands and lasts for 20–30 sec (5). Healthcare workers adopting incorrect hand hygiene techniques while using the ABHR approved by the US FDA still had detectable microorganism levels with 25% of participants achieving <1.1 log10 CFU reduction in microorganism load (27). Emphasis should, therefore, be placed on the training and enforcement of correct hand hygiene techniques with a systematic approach to application of the ABHR and allowing adequate contact time in clinical environments.
Improved hand hygiene compliance
The WHO guidelines recommend hand rubbing with ABHR for hand hygiene during patient care except when hands are visibly soiled or dirty as it is faster, more effective, and better tolerated by the skin compared to hand washing (5). The introduction of ABHR improves compliance to hand hygiene practices, thereby reducing the transmission risk of nosocomial infections. Such findings are consistent in both the critical care (8) and general ward (28) settings. Indeed, improvements in hand hygiene compliance appear to translate to better patient outcomes. For example, a multicenter study demonstrated a decrease in central line-related infections following the introduction of ABHR (29). Factors contributing to improved hand hygiene compliance include reduced skin irritation (30), improved accessibility (31), and shorter duration required to execute hand hygiene (2).
Toxicity among non-healthcare workers
Despite the benefits of alcohol-based medical products, there exists abundant literature describing toxicity resulting from the intentional or unintentional ingestion of these products. Medical and industrial products containing ethanol and isopropanol often appear similar to harmless liquids, such as water, and are commonly ingested unintentionally by children (32). Proliferation of misleading messages on social media in response to the COVID-19 pandemic resulted in the intentional ingestion of methanol by hundreds of Iranians in the belief that this would confer viral prophylaxis (33). Moreover, ABHR is frequently consumed by individuals who are alcohol dependent, especially when commercial alcohol becomes unavailable (34, 35). Finally, numerous cases of methanol poisoning from adulterated illicit alcohol have also been reported, such as in India, Indonesia, Iran, and Russia (36). The above examples highlight potential risks surrounding the use of ABHR and other medical products, particularly in the context of the ongoing COVID-19 pandemic. Disruption of supply chains and the need for increased production of medical grade alcohols might divert resources away from the production of alcoholic beverages increasing the likelihood of individuals consuming (1) ABHR and other medical products as well as (2) illicit alcohol with poor or absent quality control. The effects of either could be potentially disastrous. We propose recommendations to minimize the risk of poisoning associated with ABHR in Table 3.
Safety among healthcare workers
Unintentional absorption
Both ethanol and isopropanol have demonstrated absorption through transpulmonary and transdermal routes, and concerns exist over the unintended absorption of these substances by healthcare workers during routine use. Fortunately, the likelihood of unintended parenteral absorption of ethanol or isopropanol in healthcare workers is very low. The rise of serum and urinary levels of ethanol following the liberal usage of ABHR by healthcare workers over an 8 h shift mimics that occurring after the use of alcohol-containing products of daily life, such as aftershave and mouthwash (37). Moreover, healthcare workers did not exhibit detectable levels of alcohol in serum of urine after exposure to inhaled air with an ethanol concentration of 46.2 mg/m3 over a 4-h shift (38). Collectively, these findings suggest that transdermal and transpulmonary absorptions of ethanol and isopropanol are minimal following the use of ABHR.
Fire safety
There remains a theoretical risk of fire when using the ABHR, especially in the operating theater. A multicenter American study involving 766 health facilities using ABHR over a total of 1,430 hospital years did not reveal any incidences of fire attributable to ABHR (39). A multicenter study in Germany covering 788 hospitals reported seven non-severe cases of fire associated with ABHR (40). Three cases were related to exposure to ignition sources (cigarettes and candles) before the ABHR had fully evaporated. The remaining cases were associated with vandalism and attempted physician suicide. Similarly, an isolated flash fire was reported when static arising from personal protective equipment removal resulted in the ignition of an ABHR that had not fully evaporated (41). Despite the paucity of evidence, the risk of fire from ABHR appears low. However, health care workers are advised to take precautions to allow the alcohol hand rub to dry up completely before exposure to sources of ignition. Measures should be also taken to mitigate the fire hazard associated with ABHR. Suggestions based on recommendations from WHO (42) and the US National Fire Protection Association (NFPA) (43) are proposed in Table 4.
Conclusions
Alcohol has a longstanding history as an antiseptic. It works by denaturing protein and rendering cell membranes permeable. It offers excellent germicidal effects against Gram-positive and Gram-negative bacteria, Mycobacterium tuberculosis, fungi, and lipid-containing viruses but is ineffective against bacterial and fungal spores. ABHR usually contains 60–90% isopropanol or ethanol and additives like chlorhexidine and emollients. Besides its effective biocidal activity, ABHR is associated with an improved compliance with hand hygiene. However, there are cases of intoxications secondary to ingestion of ABHR or adulterated alcohol when resources are diverted away from the normal beverage production to meet the increased need for hand sanitizer during the COVID-19 pandemic. The risk among healthcare workers, for example, unintentional topical absorption and fire hazard, is low but should not be ignored.
Ethics approval
This review does not need ethics approval as it does not include any human or animal subjects.
References
- Mody L, Saint S, Kaufman SR, Kowalski C, Krein SL. Adoption of alcohol-based handrub by United States hospitals: a national survey. Infect Control Hosp Epidemiol 2008; 29: 1177–80. doi: 10.1086/592095
- Widmer AF. Replace hand washing with use of a waterless alcohol hand rub? Clin Infect Dis 2000; 31: 136–43. doi: 10.1086/313888
- Grayson ML, Melvani S, Druce J, Barr IG, Ballard SA, Johnson PDR, et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis 2009; 48: 285–91. doi: 10.1086/595845
- Laustsen S, Lund E, Bibby BM, Kristensen B, Thulstrup AM, Kjolseth Moller J. Effect of correctly using alcohol-based hand rub in a clinical setting. Infect Control Hosp Epidemiol 2008; 29: 954–6. doi: 10.1086/590393
- World Health Organization. WHO guidelines on hand hygiene in health care. Switzerland: World Health Organisation; 2009. Report No.: WHO/IER/PSP/2009/01.
- Pires D, Soule H, Bellissimo-Rodrigues F, Gayet-Ageron A, Pittet D. Hand hygiene with alcohol-based hand rub: how long is long enough? Infect Control Hosp Epidemiol 2017; 38: 547–52. doi: 10.1017/ice.2017.25
- Girou E, Loyeau S, Legrand P, Oppein F, Brun-Buisson C. Efficacy of handrubbing with alcohol based solution versus standard handwashing with antiseptic soap: randomised clinical trial. BMJ 2002; 325: 362. doi: 10.1136/bmj.325.7360.362
- Hugonnet S, Perneger TV, Pittet D. Alcohol-based handrub improves compliance with hand hygiene in intensive care units. Arch Intern Med 2002; 162: 1037–43. doi: 10.1001/archinte.162.9.1037
- Presterl E, Suchomel M, Eder M, Reichmann S, Lassnigg A, Graninger W, et al. Effects of alcohols, povidone-iodine and hydrogen peroxide on biofilms of Staphylococcus epidermidis. J Antimicrob Chemother 2007; 60: 417–20. doi: 10.1093/jac/dkm221
- Centers for Disease Control and Prevention, United States. Chemical disinfectants – guideline for disinfection and sterilization in healthcare facilities 2016. Available from: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html [cited 15 June 2020].
- Reichel M, Heisig P, Kohlmann T, Kampf G. Alcohols for skin antisepsis at clinically relevant skin sites. Antimicrob Agents Chemother 2009; 53: 4778–82. doi: 10.1128/AAC.00582-09
- Centers for Disease Control and Prevention, United States. Hand hygiene recommendations: guidance for healthcare providers about hand hygiene and COVID-19. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html [cited 15 June 2020].
- Centers for Disease Control and Prevention, United States. NIOSH pocket guide to chemical hazards. 2007. Available from: https://www.cdc.gov/niosh/npg/ [cited 15 June 2020].
- Food and Drug Administration, United States. Temporary policy for preparation of certain alcohol-based hand sanitizer products during the public health emergency (COVID-19) guidance for industry. 2020. Available from: https://www.fda.gov/media/136289/download. [cited 15 June 2020]
- Bonnabry P, Voss A. Hand hygiene agents. In: Pittet D, Boyce JM, Allegranzi B, eds. Hand hygiene: a handbook for medical professionals. New Jersey: John Wiley & Sons; 2017; p. 51.
- Compounding alcohol-based hand sanitizer during COVID-19 Pandemic. 2020. Available from: https://www.usp.org/sites/default/files/usp/document/about/public-policy/usp-covid19-handrub.pdf [cited 15 June 2020].
- Schülke & Mayr GmbH, Germany. Microshield handrub safety data sheet. 2015. Available from: https://www.schuelke.com/media-au-en/docs/SDS/MICROSHIELD-australia-SDS/MS-Hand-Rub.pdf [cited 15 June 2020].
- Drugs.com. Purell healthcare advanced hand sanitizer gel. 2019. Available from: https://www.drugs.com/otc/990691/purell-healthcare-advanced-hand-sanitizer-gel.html [cited 15 June 2020].
- Barbadoro P, Martini E, Savini S, Marigliano A, Ponzio E, Prospero E, et al. In vivo comparative efficacy of three surgical hand preparation agents in reducing bacterial count. J Hosp Infect 2014; 86: 64–7. doi: 10.1016/j.jhin.2013.09.013
- Aly R, Maibach HI. Comparative study on the antimicrobial effect of 0.5% chlorhexidine gluconate and 70% isopropyl alcohol on the normal flora of hands. Appl Environ Microbiol 1979; 37: 610–13. doi: 10.1128/aem.37.3.610-613.1979
- Gordin FM, Schultz ME, Huber RA, Gill JA. Reduction in nosocomial transmission of drug-resistant bacteria after introduction of an alcohol-based handrub. Infect Control Hosp Epidemiol 2005; 26: 650–3. doi: 10.1086/502596
- Kaier K, Frank U, Hagist C, Conrad A, Meyer E. The impact of antimicrobial drug consumption and alcohol-based hand rub use on the emergence and spread of extended-spectrum beta-lactamase-producing strains: a time-series analysis. J Antimicrob Chemother 2009; 63: 609–14. doi: 10.1093/jac/dkn534
- Halim MMA, Eyada IK, Tongun RM. Prevalence of multidrug drug resistant organisms and hand hygiene compliance in surgical NICU in Cairo University Specialized Pediatric Hospital. Egyptian Pediatric Association Gazette 2018; 66: 103–11. doi: 10.1016/j.epag.2018.09.003
- Latour K, Huang TD, Jans B, Berhin C, Bogaerts P, Noel A, et al. Prevalence of multidrug-resistant organisms in nursing homes in Belgium in 2015. PLoS One 2019; 14: e0214327. doi: 10.1371/journal.pone.0214327
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104: 246–51. doi: 10.1016/j.jhin.2020.01.022
- Widmer AF, Conzelmann M, Tomic M, Frei R, Stranden AM. Introducing alcohol-based hand rub for hand hygiene: the critical need for training. Infect Control Hosp Epidemiol 2007; 28: 50–4. doi: 10.1086/510788
- Widmer AE, Dangel M. Alcohol-based handrub: evaluation of technique and microbiological efficacy with international infection control professionals. Infect Control Hosp Epidemiol 2004; 25: 207–9. doi: 10.1086/502379
- Bischoff WE, Reynolds TM, Sessler CN, Edmond MB, Wenzel RP. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med 2000; 160: 1017–21. doi: 10.1001/archinte.160.7.1017
- Barrera L, Zingg W, Mendez F, Pittet D. Effectiveness of a hand hygiene promotion strategy using alcohol-based handrub in 6 intensive care units in Colombia. Am J Infect Control 2011; 39: 633–9. doi: 10.1016/j.ajic.2010.11.004
- Souweine B, Lautrette A, Aumeran C, Bénédit M, Constantin JM, Bonnard M, et al. Comparison of acceptability, skin tolerance, and compliance between handwashing and alcohol-based handrub in ICUs: results of a multicentric study. Intensive Care Med 2009; 35: 1216–24. doi: 10.1007/s00134-009-1485-5
- Giannitsioti E, Athanasia S, Antoniadou A, Fytrou H, Athanassiou K, Bourvani P, et al. Does a bed rail system of alcohol-based handrub antiseptic improve compliance of health care workers with hand hygiene? Results from a pilot study. Am J Infect Control 2009; 37: 160–3. doi: 10.1016/j.ajic.2008.04.252
- Litovitz T. The alcohols: ethanol, methanol, isopropanol, ethylene glycol. Pediatr Clin North Am 1986; 33: 311–23. doi: 10.1016/S0031-3955(16)35004-0
- Soltaninejad K. Methanol mass poisoning outbreak: a consequence of COVID-19 pandemic and misleading messages on social media. Int J Occup Environ Med 2020; 11: e1–3. doi: 10.34172/ijoem.2020.1983
- Gormley NJ, Bronstein AC, Rasimas JJ, Pao M, Wratney AT, Sun J, et al. The rising incidence of intentional ingestion of ethanol-containing hand sanitizers. Crit Care Med 2012; 40: 290–4. doi: 10.1097/CCM.0b013e31822f09c0
- Rich J, Scheife RT, Katz N, Caplan LR. Isopropyl alcohol intoxication. Arch Neurol 1990; 47: 322–4. doi: 10.1001/archneur.1990.00530030100022
- Jarwani BS, Motiani PD, Sachdev S. Study of various clinical and laboratory parameters among 178 patients affected by hooch tragedy in Ahmedabad, Gujarat (India): a single center experience. J Emerg Trauma Shock 2013; 6: 73–7. doi: 10.4103/0974-2700.110745
- Pires D, Bellissimo-Rodrigues F, Pittet D. Ethanol-based handrubs: safe for patients and health care workers. Am J Infect Control 2016; 44: 858–9. doi: 10.1016/j.ajic.2016.02.016
- Hautemaniere A, Ahmed-Lecheheb D, Cunat L, Hartemann P. Assessment of transpulmonary absorption of ethanol from alcohol-based hand rub. Am J Infect Control 2013; 41: e15–19. doi: 10.1016/j.ajic.2012.09.004
- Boyce JM, Pearson ML. Low frequency of fires from alcohol-based hand rub dispensers in healthcare facilities. Infect Control Hosp Epidemiol 2003; 24: 618–19. doi: 10.1086/502262
- Kramer A, Kampf G. Hand rub-associated fire incidents during 25,038 hospital-years in Germany. Infect Control Hosp Epidemiol 2007; 28: 745–6. doi: 10.1086/517953
- Bryant KA, Pearce J, Stover B. Flash fire associated with the use of alcohol-based antiseptic agent. Am J Infect Control 2002; 30: 256–7. doi: 10.1067/mic.2002.125395
- World Health Organization. Alcohol-based handrub risks/hazards. 2020. Available from: https://www.who.int/gpsc/tools/faqs/abhr2/en/ [cited 28 June 2020].
- National Fire Protection Association, United States. NFPA 101 life safety code. 2018 ed. 2018. Available from: https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=101 [cited 28 June 2020].
- World Health Organization. WHO guidelines on hand hygiene in health care (WHO/IER/PSP/2009/01). 2009. Available from: https://www.who.int/gpsc/information_centre/hand-hygiene-2009/en/ [cited 15 June 2020].