ORIGINAL ARTICLE
Feasibility and safety of a symptom-based strategy to discontinue infection control precautions for patients hospitalised with COVID-19 – a retrospective study
Gaël Grandmaison1#*, Marine Baumberger1#, Charlotte Pellaud1, Véronique Erard2 and Christian Chuard2
1Division of internal Medicine, HFR Fribourg, Fribourg, Switzerland; 2Division of Infectiology, HFR Fribourg, Fribourg, Switzerland
# Authors contributed equally to work
Abstract
Background: Various recommendations exist concerning the discontinuation of contact and droplet precautions (CDP) for patients hospitalised with coronavirus disease 2019 (COVID-19). Some are based on repeated negative real-time polymerase chain reaction (RT-PCR) results, whereas other are based on clinical criteria. The feasibility and safety of these recommendations are poorly documented.
Method: We conducted a retrospective study to assess the feasibility and safety of a symptom-based strategy to discontinue CDP for patients hospitalised with COVID-19. We reviewed the clinical charts of all symptomatic patients hospitalised in our institution with RT-PCR-confirmed COVID-19 to assess the application of a symptom-based strategy for the implementation and discontinuation of CDP. The patients with discontinuation of CDP in accordance with the symptom-based strategy were cross-referenced with patients with potential hospital-acquired COVID-19 in order to assess the safety of this strategy.
Results: Among the 147 patients included in our study, our symptom-based strategy was respected in 95 cases (64.6%). Discontinuation of CDP in accordance with the recommendations occurred in 39 patients (26.5%). After the discontinuation of CDP, patients remained hospitalised for a median time of 18 days, with exposure to a median number of three patients, resulting in a total number of 588 days ‘patient-day-exposition’. No hospital-acquired COVID-19 was detected in contact patients.
Discussion: The use of a symptom-based strategy to discontinue CDP is applicable and safe. This symptom-based strategy was applicable regardless of patient’s age or COVID-19 severity.
Keywords: COVID-19; infection control; hospitals; feasibility studies; symptom-based strategy; Switzerland
Citation: Int J Infect Control 2021, 17: 20601 – http://dx.doi.org/10.3396/ijic.v17.20601
Copyright: © 2021 Gaël Grandmaison et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 16 July 2020; Accepted: 27 July 2020; Published: 31 August 2021
Competing interests and funding: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
*Gaël Grandmaison, Chemin des Pensionnats 2, 1708 Fribourg, Switzerland. Email: Gael.Grandmaison@h-fr.ch
In this article, we reviwed the clinical chart of all hospitalised patients with real-time polymerase chain reaction (RT-PCR)-confirmed COVID-19 to assess the application of a symptom-based strategy for discontinuation of additional infection control precautions. To assess the safety of this strategy, we cross-referenced the patients still hospitalised after contact and droplet precautions (CDP) discontinuation in accordance with recommendations for patients who had potentially developed hospital-acquired COVID-19.
Background
Since its first description in December 2019 in Wuhan, China (1), coronavirus disease 2019 (COVID-19) has quickly spread throughout the world. The hospitalisation rate for COVID-19 patients is estimated to be about 20% (2).
Transmission of COVID-19 occurs primarily through close-range contact and droplets (3). The World Health Orginization currently recommends contact and droplet precautions (CDPs) for patients with suspected or confirmed COVID-19, while applying airborne precautions when performing aerosol-generating procedures (3). CDP is essential to prevent cross-transmission of the infection between patients and healthcare workers. However, in order to best allocate resources and optimise patient care, it is essential to discontinue CDP as soon as patients are no longer contagious (4–6).
Various recommendations exist concerning the discontinuation of CDP in the hospital setting. Some require repeated negative RT-PCR results (test-based strategy), whereas others are based on clinical criteria such as the resolution of symptoms and the duration since the beginning of the illness (symptom-based strategy) (7–9). However, the feasibility and safety of these recommendations are poorly documented.
During the beginning of the COVID-19 epidemic, we implemented additional infection control precautions using a symptom-based strategy in accordance with national recommendations (www.swissnoso.ch). Additional infection control precautions consisted of CDP for at least 10 days after onset of symptoms and 2 days without symptoms. In addition, airborne precaution was required for aerosol-generating procedures.
The purpose of this article is to describe the feasibility and safety of this symptom-based strategy to discontinue CDP during hospitalisation.
Method
We conducted a retrospective study in a network of public hospitals in the area of Fribourg, Switzerland. This network consists of five hospitals with one intensive care unit (ICU), four acute medical care wards and four rehabilitation wards.
We reviewed the clinical chart of all patients hospitalised with RT-PCR-confirmed COVID-19 between 1 March 2020 and 12 April 2020 to document the implementation of CDP. During this period, the discontinuation of CDP was determined using a symptom-based strategy. In order to discontinue CDP, patients had to meet both of the following criteria: at least 10 days elapsed since the appearance of first symptoms and at least 2 days without symptoms. The same strategy was applied regardless of the severity of the disease. After discontinuation of the CDP, standard precautions were applied.
Patients under 18 years of age, patients transferred to other hospitals and patients who were discharged home less than 10 days after appearance of symptoms were excluded from the study. Patients with CDP extended after 23 April 2020 were also excluded due to the modification of the symptom-based strategy rules after this date.
For each patient, demographic data, comorbidities and severity of illness were documented. The severity of COVID-19 was defined according to WHO criteria (10).
In order to assess the applicability of the precited recommendations, electronic health records were reviewed to determine the adequacy of either CDP prolongation or discontinuation 10 days after symptoms onset. Reasons for prolongation or discontinuation of the CDP were documented. The implementation of CDP was considered appropriate, provided the national recommendations were respected, while considered inappropriate if they were not. The extension of CDP beyond 10 days because of the absence of clinical improvement, persistent symptoms (defined as fever and diarrhoea), need of oxygen support >4 L/min, or ongoing ICU stay was considered as appropriate. The extension of CDP was considered inappropriate if justified only by the persistence of nonspecific symptoms (defined as dyspnoea or cough persistent after clinical improvement), need of oxygen support <4 L/min, for practical reasons (waiting for discharge or waiting for transfer or delay due to weekend), or because of aerosol-generating procedures (tracheostomy or non-invasive ventilation).
The safety of our CDP policy was established by cross-referencing COVID-19 patients still hospitalised after discontinuation of CDP in accordance with the recommendations for patients who had potentially developed a hospital-acquired COVID-19. For this purpose, all hospitalised patients who suffered onset of COVID-19 symptoms after admission were considered as having a potential hospital-acquired infection. In order to have sufficient follow-up, we continued to look for potential hospital-acquired infections up to 30 days after the last CDP discontinuation.
We present continuous variables as median and interquartile range (IQR) and categorical variables as number and percentage.
Results
During the study period, we identified 201 inpatients with PCR-confirmed COVID-19. Among them, we excluded one patient for being under 18 years old, three transferred to other hospitals, 44 discharged home less than 10 days after symptoms onset and six with CDP extended beyond 23 April 2020 (Fig. 1).
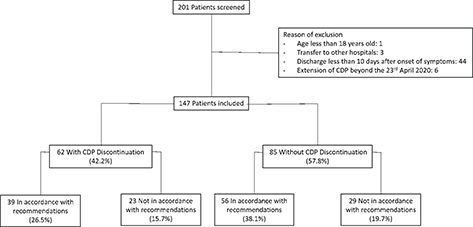
Fig. 1. Flow chart.
CDP = contact and droplet precautions.
There were 147 patients eligible for the study. The median age was 71 years old (IQR 61–79) and 89 patients (60.5%) were men. Forty-one patients (27.9%) required ICU, and 27 (18.4%) required invasive mechanical ventilation. The time between symptoms onset and hospitalisation was 8 days (IQR 4–10). The median in-hospital stay duration was 10 days (IQR 6–15.5). The median duration of CDP was 9 days (IQR 6–13). The comorbidities and the severity of COVID-19 are presented in Table 1. Among these patients, the additional precautions were discontinued during hospital stay in 62 cases (42.2%) (Fig. 1). The characteristics of patients with discontinuation and without discontinuation of CDP are presented in Table 1. Patients for whom CDP were discontinued were found to be older, had longer hospital stay and higher need of intensive care or invasive mechanical ventilation.
Of the 62 cases of CDP discontinuation, 39 cases (26.5%) occurred in accordance with recommendations (Fig. 1). For these latter patients, the median length of CDP was 13 days (IQR 10–18.5). The delay between onset of symptoms and the day of discontinuation of CDP was 19 days (IQR 13–24.5). Only six patients were no longer symptomatic 10 days after onset of symptoms. The reasons for the adequate prolongation of CDP were mainly ongoing ICU stay and persistent symptoms (Table 2).
| With CDP Discontinuation (n) | Without CDP Discontinuation (n) | |
| Recommendations respected | 33* | 56 |
| - Persistent symptoms | 33 | 56 |
| - Ongoing intensive care unit (ICU) stay | 16 | NA |
| - Fever | 9 | 22 |
| - Diarrhoea | 7 | 9 |
| - Oxygen support >4 L/min | 1 | 4 |
| - Symptoms until death | 0 | 21 |
| Recommendations non-respected | 23 | 29 |
| - Practical reasons | 6 | 16 |
| - Awaiting discharge | NA | 15 |
| - Awaiting transfer | 4 | 0 |
| - Weekend | 2 | 1 |
| Persistent non-specific symptoms | 16 | 13 |
| - Oxygen therapy < 4 L/min | 10 | 4 |
| - Low grade fever | 4 | 5 |
| - Cough | 2 | 2 |
| - Confusion state | 0 | 1 |
| - Asthenia | 0 | 1 |
| Aerosol generating procedures | 1 | 0 |
| - Non-invasive ventilation | 1 | 0 |
| *Six additional patients were asymptomatic 10 days after onset of symptoms and benefited from CDP discontinuation in accordance with recommendations. NA, not applicable. | ||
In 23 cases (15.7%), CDP was discontinued beyond the length of time defined by the recommendations. The delay between onset of symptoms and discontinuation of CDP was 14 days (IQR 12–18.5). The median duration of CDP was 11 days (IQR 9.5–13). Delay between the theoretical date and the effective date of CDP discontinuation was 3 days (IQR 1.5–4), ranging from 1 to 8 days. The reasons for inappropriate prolongation of CDP were mainly persistent nonspecific symptoms and are detailed in Table 2.
Among the 85 patients for whom CDP was maintained during the entire hospital stay, 56 cases (38.1%) were in accordance with recommendations. For these patients, prolongation of CDP until discharge from hospital was justified by prolonged symptoms, mainly fever and diarrhoea (Table 2). In 21 cases, CDP was prolonged until death in accordance with recommendations. In 29 cases (19.7%), CDP was extended regardless of recommendations. The main reasons were practical reasons and persistent nonspecific symptoms (Table 2). The median delay between theoretical discontinuation of CDP and effective time of discharge from hospital was 3 days (IQR 2–5), ranging from 1 to 8 days.
Overall, the symptom-based strategy was respected in 95 cases (64.6%). The characteristics of patients with respect or without respect of the recommendations are presented in Table 3. CDP discontinuation rate was similar in both groups. The recommendations were more frequently followed in younger patients, patients with more severe COVID-19 and patients needing ICU stay or invasive mechanical ventilation.
After discontinuation of CDP in accordance with recommendations, the 39 post-COVID-19 patients remained hospitalised for a median time of 18 days (IQR 9.5–27.5) and shared their room with a median number of three patients (IQR 1–4). The total number of patient-day-exposure amounted to 588 days. During this exposure period, none of the contact patients developed a potential hospital-acquired COVID-19 infection.
Discussion
Our study demonstrates the feasibility and safety of a symptom-based strategy to guide discontinuation of CDP in the hospital setting. The symptom-based strategy was correctly applied in 95/147 cases (64.6%) and led to the discontinuation of CDP in 62 cases (42.2%). Respect of the symptom-based strategy was more frequent in patients with longer in-hospital stay. Following discontinuation of CDP, patients remained hospitalised with other patients without the implementation of supplementary infection control precautions. The absence of hospital-acquired COVID-19 among the contact patients exposed in the same room of these patients confirms the safety of this strategy.
These data are reassuring since these patients remained hospitalised for an average of 18 days after the end of CDP and were hospitalised in the same rooms with patients not suffering from COVID-19. Follow-up of patients in rehabilitation wards and search of potential hospital-acquired COVID-19 until 30 days after the last CDP discontinuation improved the reliability of our results.
Some studies report a longer viral shedding in older patients and in more severe infections, suggesting the need for more stringent precautions in these populations (11). In our study, the same strategy has been applied regardless of age or severity of disease. Furthermore, discontinuation of CDP complied more frequently with the recommendations in older patients and in more severe COVID-19. Consequently, we believe that this symptom-based strategy is applicable to all COVID-19 hospitalised patients. However, it must be specified that patients with ongoing ICU stay were considered to have symptoms justifying a prolongation of CDP until their discharge from ICU. Therefore, applicability of this strategy to patients with ongoing ICU stay must be reserved. Moreover, some authors suggested a longer infectivity in immunocompromised patients (12). In our study, the few immunocompromised patients make it impossible to draw conclusion about this subpopulation.
The definition of a symptom-free status constitutes a challenge in applying a symptom-based strategy. The Swiss recommendations do not specify symptoms justifying the prolongation of CDP. This can lead to variability in the interpretation of the recommendations. In order to assess whether or not the recommendations had been followed, we defined a posteriori the symptoms justifying the extension of CDP. The relative low adherence to the recommendations seems mainly explained by the variation in the interpretation of symptoms justifying the extension of CDP. This variability explained the extension of CDP beyond the recommended time in most of the cases.
If the application of CDP is essential to prevent the transmission of COVID-19 within hospitals (3), it is also important to be able to discontinue them as quickly as possible. Unnecessary prolonged CDP is associated with an increase in the use of personal protective equipment, with less favourable outcomes (5), an increased duration of stay and costs (6). Hospital strategy should allow prompt discontinuation of additional infection control precautions without increasing the risk of transmission of COVID-19. There are currently no data in the literature comparing symptom-based and test-based strategies to discontinue the CDP. We cannot rule out the possibility that a test-based strategy may shorten the duration of CDP. However, a test-based strategy could present several limitations. First, it has been shown that patients can have a prolonged RNA shedding up to 4 weeks after a COVID-19 without a correlation with contagiousness (13–16). Second, the sensitivity of PCR testing remains imperfect and could lead to the premature discontinuation of CDP (17, 18). Third, the systematic use of RT-PCR prior to discontinuation of CDP results in the consumption of large amounts of testing material (swabs and tests), sometimes in shortage, and an increase in the costs. Finally, the safety of a test-based strategy and the gain in reducing the duration of CDP remains to be demonstrated (15, 19).
The application of a symptom-based strategy resulted in discontinuation of CDP in 62 cases (42.2%) with a median time of 19 days after onset of symptoms and 13 days after admission to hospital. Data from the literature suggest neither virus replication nor infectivity more than 10 days after onset of symptoms (13–15, 20). The applied symptom-based strategy resulted in a much longer duration of CDP.
Therefore, the necessity to prolong CDP until 48 h after resolution of symptoms could be assessed, and the possibility of discontinuing the CDP is based solely on time elapsed since the symptoms’ onset should be studied.
Our study has several limitations. First, the criteria of the symptom-based strategy are highly subjective. Therefore, the assessment of compliance with this strategy may be subject to discussion. We have been very restrictive regarding the symptoms justifying the extension of CDP. Less restrictive criteria may have increased the level of compliance with the recommendations. Second, we considered that the stay in ICU was a criterion justifying the prolongation of CDP. Therefore, although criteria for CDP discontinuation were applied at discharge from ICU, we cannot comment on the applicability and safety of these criteria for patients during an ICU stay. Finally, we were not able to demonstrate that there was no transmission of COVID-19 to the healthcare workers after discontinuation of CDP. However, no cluster of COVID-19 was reported among healthcare workers in our institution during the studied period. Furthermore, it would be difficult to conclude whether COVID-19 in a healthcare worker would be attributable to contact with a patient or to infection outside the hospital.
In conclusion, we have shown that the use of a symptom-based strategy to discontinue CDP within hospitals is applicable and safe. The strategy has been applied to all patients, regardless of age or disease severity.
Declaration
Ethics approval and consent to participate
This work was approved by our institutional board. We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. All authors have read and approved the submission of the manuscript.
Acknowledgements
Manuscript preparation: Datawarehouse, Intelligence Décisionnelle et Statistiques HFR provided assistance with data acquisition.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. doi: 10.1056/NEJMoa2001017
- Wu Z, McGoocan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. doi: 10.1001/jama.2020.2648
- World Health Organization. Clinical management of COVID-19, Interim guidance 27 May 2020. WHO website. 2020. Available from: https://apps.who.int/iris/handle/10665/332196 [cited 5 June 2020].
- The Lancet. COVID-19: protecting health care workers. Lancet 2020; 395: 922. doi: 10.1016/S0140-6736(20)30644-9
- Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control 2009; 37: 85–93. doi: 10.1016/j.ajic.2008.04.257
- Tran K, Bell C, Stall N, Tomlinson G, McGeer A, Morris A, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med 2016; 32: 262–8. doi: 10.1007/s11606-016-3862-4
- Public Health England. Guidance for stepdown of infection control precautions and discharging COVID-19 patients. UK Government website. 2020. Available from: https://www.gov.uk/government/publications/covid-19-guidance-for-stepdown-of-infection-control-precautions-within-hospitals-and-discharging-covid-19-patients-from-hospital-to-home-settings [cited 6 June 2020].
- Health Protection Scotland. COVID-19 – guidance for stepdown of infection control precautions and discharging COVID-19 patients from hospital to residential settings. HPS website. 2020. Available from: https://www.hps.scot.nhs.uk/web-resources-container/covid-19-guidance-for-stepdown-of-infection-control-precautions-and-discharging-covid-19-patients-from-hospital-to-residential-settings/[cited 6 June 2020].
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Discontinuation of transmission-based precautions and disposition of patients with COVID-19 in healthcare settings (interim guidance). Center for Disease Control and Prevention website. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html [cited 6 June 2020].
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. 2020. Available from: https://apps.who.int/iris/handle/10665/331446 [cited 15 May 2020].
- Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhjiang province, China, January-March 2020: a retrospective cohort study. BMJ 2020; 369: m1443. doi: 10.1136/bmj.m1443
- Leonard AM. Disposition of patients with COVID-19 infection whose respiratory specimens remain positive for SARS-CoV-2 by PCR. Infect Contr Hosp Epidemiol 2020; 41(11): 1326–7.
- Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious SARS-CoV2 from diagnostic samples. Clin Infect Dis 2020 ; Volume 71, Issue 10, 15 November 2020, Pages 2663–6.
- Wöfel R, Corman VM, Guggemos W, Seilmaier, Zange S, Müller, MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–9. doi: 10.1038/s41586-020-2196-x
- Kujawski SA, Wong KK, Collins JP, Epstein L, Killerby ME, Midgley CM, et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med 2020; 26: 861–8. doi: 10.1038/s41591-020-0877-5
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. doi: 10.1016/S0140-6736(20)30566-3
- Kurcika LM, Lauer SA, Layendecker O, Boon D, Lessler J. Variation in false-negative of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020; 173(4): 262–7. doi: 10.7326/M20-1495
- Suzuki T, Kutsuna S, Nakamura K, Ide S, Moriyama Y, Saito S, et al. Difficulty of downscaling the precautions for coronavirus disease-19 based on negative throat polymerase chain results in the early phase of infection. J Infect Chemother 2020; 26: 851–3. doi: 10.1016/j.jiac.2020.05.002
- La Scola B, Le Bideau M, Andreani J, Hoang VT, Grimaldier C, Colson P, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020; 39: 1059–61. doi: 10.1007/s10096-020-03913-9
- Cheng H-Y, Jian S-W, Liu D-P, Ng T-C, Huang W-T, Lin H-H. Contact tracing assessment of COVID-19 transmission dynamics in Taïwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020: e202020. doi: 10.1001/jamainternmed.2020.2020