ORIGINAL ARTICLE
Improving hand hygiene measures in low-resourced intensive care units: experience at the Kigali University Teaching Hospital in Rwanda
Jean Paul Mvukiyehe1*, Eugene Tuyishime1, Anne Ndindwanimana2, Jennifer Rickard3,4, Olivier Manzi5, Gregory R. Madden6, Marcel E. Durieux7 and Paulin R. Banguti1
1Department of Anesthesia, Critical Care and Emergency Medicine, University of Rwanda, Kigali, Rwanda; 2Department of General Medicine, University of Rwanda, Kigali, Rwanda; 3Department of Surgery, University of Minnesota, Minnesota, USA; 4Department of Surgery, University Teaching Hospital of Kigali, Kigali, Rwanda; 5Department of Internal Medicine, Kigali University Teaching Hospital, Kigali, Rwanda; 6Department of Internal Medicine, Division of Infectious Diseases, University of Virginia, Charlottesville, USA; 7Department of Anesthesiology, University of Virginia, Charlottesville, USA
Abstract
Background: Proper hand hygiene (HH) practices have been shown to reduce healthcare-acquired infections. Several potential challenges in low-income countries might limit the feasibility of effective HH, including preexisting knowledge gaps and staffing.
Aim: We sought to evaluate the feasibility of the implementation of effective HH practice at a teaching hospital in Rwanda.
Methods: We conducted a prospective quality improvement project in the intensive care unit (ICU) at the Kigali University Teaching Hospital. We collected data before and after an intervention focused on HH adherence as defined by the World Health Organization ‘5 Moments for Hand Hygiene’ and assuring availability of HH supplies. Pre-intervention data were collected throughout July 2019, and HH measures were implemented in August 2019. Post-implementation data were collected following a 3-month wash-in.
Results: In total, 902 HH observations were performed to assess pre-intervention adherence and 903 observations post-intervention adherence. Overall, HH adherence increased from 25% (222 of 902 moments) before intervention to 75% (677 of 903 moments) after intervention (P < 0.001). Improvement was seen among all health professionals (nurses: 19–74%, residents: 23–74%, consultants: 29–76%).
Conclusions: Effective HH measures are feasible in an ICU in a low-income country. Ensuring availability of supplies and training appears key to effective HH practices.
Keywords: hand hygiene; hospitals; intensive care units; less-developed countries; Rwanda
Citation: Int J Infect Control 2021, 17: 20585 – http://dx.doi.org/10.3396/ijic.v17.20585
Copyright: © 2021 Jean Paul Mvukiyehe et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 9 July 2020; Accepted: 7 August 2020; Published: 10 September 2021
Competing interests and funding: The authors report no conflicts of interest. This work was supported by the UVA Global Infectious Diseases Institute, National Institutes of Health Center for Advancing Translational Science (UL1TR003015/KL2TR003016), and National Institute Of Allergy And Infectious Diseases (K23AI163368).
*Jean Paul Mvukiyehe, Department of Anesthesia, Critical Care and Emergency, Medicine, University of Rwanda, Kigali, Rwanda. Email: mvukipaul@gmail.com
Intensive care units (ICUs) are magnets for drug-resistant organisms. Patients in the ICU are highly susceptible to healthcare-acquired infections (HAIs) due to severity of illness, frequent and intense healthcare worker contact, antimicrobial use, invasive medical devices, and close proximity to infected patients. HAIs are the leading cause for the emergence and spread of multidrug resistant pathogens. Broad spectrum antibiotics are used more frequently in ICUs than in other hospital departments. Thus, drug-resistant organisms are more likely to be present (1–3).
Epidemiological data from low- and middle-income countries (LMICs) show that antimicrobial resistance (AMR) is a substantial threat, with major economic and health consequences (4). Both the World Health Organization (WHO) when discussing the global status of AMR (5) and the ‘Review on Antimicrobial Resistance’ commissioned by the UK government (6) characterize AMR as an issue of global importance. A previous study done at the Kigali University Teaching Hospital (KUTH) ICU showed that sepsis was the most common diagnosis (34%). Organisms isolated most frequently included Klebsiella species and Acinetobacter species with high resistance levels to third-generation cephalosporins (7).
HAIs are more common in LMICs than in high-income countries (HICs). The incidence of HAIs in ICUs in LMIC is estimated to be at least three times higher (47.9 per 1,000 patient-days; 95% CI, 36.7–59.1) than that reported from the United States (8).
Hand hygiene (HH) measures as defined by WHO guidelines (9) are effective in reducing HAIs in HICs. Use of alcohol hand rub reduces HAIs by 36% (10, 11), and various studies indicate that HH measures are a cost-effective method of preventing HAIs (12, 13). However, it is far from certain that such HH interventions would be feasible and effective in LMICs. Many such settings lack an established culture of infection control with associated systems and funding. Hospital infection control committees may not focus attention on the ICU, and HH measures in critically ill patients often are not assessed or enforced. Hospital infection control efforts in low-income countries are hindered by a lack of awareness of the problem, lack of personnel, poor water supply, erratic electricity supply, ineffective antibiotic stewardship policies, and poor laboratory resources (14). Frequently, no identified champions are available to take a leadership role in the ICU. Many facilities in LMICs have limited human resources, low staff to patient ratios, and thus each staff member will be contacting many more patients than would be the case in a HIC ICU. This makes the need for scrupulous HH arguably more important in LMICs, while at the same time, understaffing increases time pressure so that HH measures may be skipped. Compounding these issues is a chronic and often profound shortage of even basic infection prevention materials such as alcohol gel and gloves. For all these reasons, it cannot be assumed that HH approaches that have been shown effective in HIC settings will be feasible in LMIC ICUs.
Substantial evidence from high-income settings demonstrates that HH measures can be implemented easily and are extremely effective. However, surprisingly few studies have evaluated the feasibility and efficacy of basic HH measures in LMICs, despite the major implications for approaching ICU infection prevention as a whole in LMICs. We hypothesized that HH practices can be effectively introduced with high compliance in an LMIC ICU. We investigated the feasibility of implementing an effective HH practice infection control training bundle and assuring availability of HH supplies and studied the effects on provider behavior at a representative low-income country (LIC) ICU at KUTH in Rwanda.
Methods
We conducted a prospective observational study of the implementation of a HH bundle at the KUTH ICU. KUTH is a public tertiary referral and teaching hospital with approximately 540 beds located in the capital of Rwanda, Kigali. It has an emergency department, inpatient internal medicine, surgery, pediatrics, obstetrics and gynecology wards, and ICU. Its catchment area includes more than 19 district hospitals in Kigali and the adjacent Northern and Southern provinces.
The ICU has seven adult ICU beds and an adjacent high-dependency (step-down) unit with four adult beds. All 11 beds were studied in this project. The unit is staffed by a total of six anesthesiologists, five of whom were present during the implementation. One anesthesiologist staffs the ICU each week and is supported by two to three residents from both anesthesiology and surgery departments. A total of 33 nurses work in the ICU, six or seven of whom are present during the day shift, each caring for one to three patients. In addition, one nutritionist and two aides support the unit, and nursing and medical students rotate there.
On average, the ICU admits 25–35 patients per month with surgical (including trauma), neurosurgical, medical, and obstetrical conditions requiring intensive care. Occasionally, pediatric patients are admitted when the three-bed pediatric ICU is full.
The study was performed in three phases.
Phase 1
Pre-intervention. The aim was to target a large sample size of around 900 observations in pre- and post-interventions based on a literature review of similar studies (15–17). We collected pre-intervention data on HH adherence from July 18 to August 9, 2019 by using the WHO ‘My 5 Moments of Hand Hygiene’: before patient contact, before aseptic technique, after body fluid risks, after patient contact, and after contact with patient surroundings (18, 19). HH was primarily performed using a water basin and soap stationed in the ICU, in addition to supplies of alcohol gel distributed to each patient’s bed to mitigate frequent shortages of water. Dedicated research personnel were trained on how to use the WHO 5 Moments of Hand Hygiene observation tool (two sessions of 1 h each) and stationed in the ICU to observe and audit compliance with HH practices. Research personnel evaluated providers at each opportunity to interact with patients during at least three 8-h periods each week. Availability of hand washing supplies (water, soap, and alcohol) and their uses relating to WHO 5 moments were also assessed.
Phase 2
Intervention. We implemented proper HH according to WHO HH recommendations. In August 2019, we organized short, in-person training with two sessions (3 h each) for training on proper HH focused on the WHO 5 Moments. Infection prevention and control bundle courses in task-appropriate infection control measures were organized for all personnel with patient access in the ICU: physicians, nurses, aides, nutritionist, students at various levels, and support personnel. In order to optimize inter-auditor reproducibility, training sessions incorporated observation and scoring of standardized patient care episodes according to WHO guidelines, with feedback on accuracy and consistency. Cognitive aids in the form of posters and handouts were prepared and distributed. Availability of HH materials (alcohol gel, etc.) was ensured.
Phase 3
Post-intervention. After a 3-month ‘wash-in’ period, we collected post-intervention data from December 1, 2019 to January 9, 2020 using the same methodology as during the pre-intervention phase. Observers were trained for two sessions of 1 h each on how to use a WHO 5 Moments observation tool. Observers audited again as in Phase 1. The data collection form was created in Epi Info 7.0 (Centers for Disease Control and Prevention [CDC], Georgia, USA) using the WHO 5 Moments observation tool. The ICU health workers were aware of pre- and post-intervention data collection.
Statistical Analysis
Data were collected and analyzed using Epi Info 7.0 (CDC) and Microsoft Excel (Microsoft Corporation, Washington, USA). We calculated descriptive statistics. Comparisons were made by Chi square test. P < 0.05 was considered statistically significant.
Results
In total, 902 HH observations were performed pre-intervention and 903 observations post-intervention. Overall, HH adherence tripled from 25% (222 of 902 moments) pre-intervention to 75% (677 of 903 moments) post-intervention (P < 0.001). Substantial improvements were noted for each of the WHO ‘5 Moments for Hand Hygiene’ opportunities (Fig. 1). The greatest improvement was noted before patient contact (absolute difference 58%); the smallest improvement was noted after patient contact (absolute difference 34%). All staff categories showed more than 70% compliance after intervention. Improvement was seen in each staff category (Fig. 2) with greatest improvement in medical students (absolute difference of 79%) and the smallest improvement in nursing students (absolute difference of 43%). Availability of alcohol-based hand wash increased from 76% to 99%.
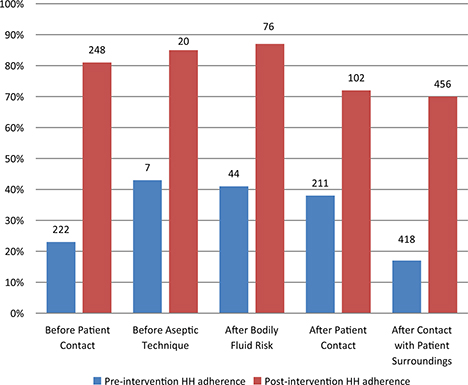
Fig. 1. Adherence with World Health Organization’s ‘My 5 moments for Hand Hygiene’ before and after intervention. Number above bars indicates the number of observations for each group.
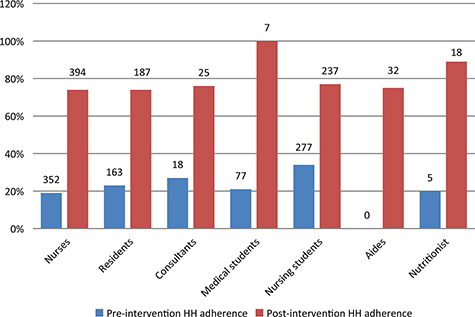
Fig. 2. Adherence to hand hygiene protocols by staff group before and after intervention. Number above bars indicates the number of observations for each group.
Discussion
Our results suggest that the implementation of effective HH measures in ICUs of LMICs is as feasible as it is in higher-resourced settings. Although not a focus of our study, other studies in HICs have associated the implementation of HH with reductions in HAIs (9), suggesting that the same might be the case in LMICs. The important factors were engagement of all personnel, focused training with reminders in the form of cognitive aids, and availability of HH materials, which include a regular supply of water, soap, gloves, and alcohol gel.
This ICU has two main water sinks for hand washing, but frequent water cut-offs occur. During the study period from pre-implementation to post-implementation, we ensured the presence of other HH materials like alcohol gel, gloves, paper towels, etc. We involved the hospital leadership to continue supplying these HH materials.
Although direct comparison of auditing practices is difficult, HH adherence in response to our education-based intervention appeared greater than in a similar multicenter collaborative study in the United States using product/volume usage measurement and feedback; in that study, the baseline ICU HH compliance increased from 26% to only 51% (20). In our setting, despite limited resources and low staff to patient ratio, HH compliance tripled.
Our pre-intervention findings were concordant with those of a systematic review, which found that baseline HH compliance is high in HICs at 64.5% but much lower in LICS (9–32%) (15, 21). The magnitude of improvement with an education-based HH program was near that of a study done at Swiss multisite regional hospital, where compliance increased from 61.4% to 83.4% after 18 months of the program (16). The large effect size seen in this study may be explained by a preexisting knowledge gap and lack of regular supplies of infection control materials, such as alcohol gel for hand cleaning and factors that are common in LMICs (22, 23). There remains a great need to emphasize infection prevention and control measures in LMIC nursing and medical schools (24, 25).
Although we did not investigate the impact of our intervention on HAIs specifically, other studies have shown that a decrease in HAI is a likely outcome of improved HH. A study in Vietnam in a low-resourced ICU studied the effect of proper HH adherence on HAI and cost of health care. HH adherence doubled from pre- to post-intervention (25.7–57.5%). This was associated with a reduction of HAI (from 31.7% to 20.3%) and substantial cost savings of $1,074 for each HAI prevented (26).
Studies have found that health workers are concerned about exposure to diseases after procedures. This may explain the high overall compliance after patient contact in our study (27). We found post-intervention HH adherence to be the highest in medical students and the nutritionist (only one nutritionist works at this unit). This finding contrasts with other studies, where compliance was higher among nurses (43%) than physicians (19%) and other health care workers (28%) (28, 29).
LMICs often are unable to invest in sufficient facilities for HH, thus creating a barrier for improving HH adherence. Therefore, for our implementation, we focused on basic, low-cost WHO-recommended steps (providing HH facilities, training, surveillance, and feedback) (30, 31).
Several limitations of our study should be recognized. Our ICU admits medical, surgical, and obstetrical patients, and as a result, residents and consultants from those departments make rounds in the unit. These personnel did not participate in the teaching and training but were audited during the pre- and post-intervention phases. This may have led to bias among health professional HH adherence, but if anything, it likely would have reduced any improvement noted. Another important consideration is that audits were performed only during regular day shifts, that is, Monday-Friday up to 7 pm. Further studies could monitor HH adherence during night shifts and weekends. We cannot rule out a study effect caused by the presence of audit personnel in the ICU. We tried to mitigate this as much as possible by waiting several months between training and post-intervention audits. Also, any study effect likely would have been similar during pre- and post-intervention periods, if not more pronounced during the pre-intervention period. Finally, we do not know if the effects observed will be sustained over longer time periods without repeat training.
We conclude from our findings that instituting effective HH measures is feasible and associated with high compliance in an ICU of an LIC. Our data suggest that regular supplies of infection control materials and training are key to effective HH practice. Further research is recommended to study any changes in HAI and multidrug resistant infections after these interventions.
Acknowledgments
We would like to thank the Global Infectious Disease Institute at the University of Virginia and Kigali University Teaching Hospital for financial support of this project. We would also like to thank the students from the University of Virginia who assisted in data collection: Sabrina Cumpian, Mattie Hyler, Caroline Porco, and Elle Worley.
Financial disclosures
None.
Ethics statement
The study was approved by the College of Medicine and Health Sciences’ Institutional Review Board, No. 150/CMHS IRB/2019, and KUTH, Ref: EC/CHUK/0118/2019. Requirement for written documentation of informed consent was waived by the institutional review board.
References
- Dondorp AM, Limmathurotsakul D, Ashley EA. What’s wrong in the control of antimicrobial resistance in critically ill patients from low- and middle-income countries? Intensive Care Med 2018; 44(1): 79–82. doi: 10.1007/s00134-017-4795-z
- Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol 2014; 17(8): 552–7. doi: 10.2307/30141291
- Laupland KB, Zygun DA, Doig CJ, Bagshaw SM, Svenson LW, Fick GH. One-year mortality of bloodstream infection-associated sepsis and septic shock among patients presenting to a regional critical care system. Intensive Care Med 2005; 31(2): 213–19. doi: 10.1007/s00134-004-2544-6
- Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 2012; 40(5): 396–407. doi: 10.1016/j.ajic.2011.05.020
- World Health Organization. Antimicorbial resistence global report on surveillance. Available from: https://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/ [cited 27 July 2020].
- The review on antimicrobial resistance chaired by J O’Neill. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf [cited 27 July 2020].
- Mvukiyehe JP. Infections in a tertiary referral hospital intensive care unit in Rwanda. Am J Infect Control 2018; 46(6): S20–21. doi: 10.1016/j.ajic.2018.04.068
- Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011; 377(9761): 228–41. doi: 10.1016/S0140-6736(10)61458-4
- World Health Organisation. WHO guidelines on hand hygiene in health care: first global patient safety challenge – Clean care is safer care. Available from: https://www.who.int/gpsc/5may/tools/9789241597906/en/ [cited 27 July 2020].
- Souweine B, Lautrette A, Aumeran C, et al. Comparison of acceptability, skin tolerance, and compliance between handwashing and alcohol-based handrub in ICUs: results of a multicentric study. Intensive Care Med 2009; 35(7): 1216–24. doi: 10.1007/s00134-009-1485-5
- Hilburn J, Hammond BS, Fendler EJ, Groziak PA. Use of alcohol hand sanitizer as an infection control strategy in an acute care facility. Am J Infect Control 2003; 31(2): 109–16. doi: 10.1067/mic.2003.15
- Chen YC, Sheng WH, Wang JT, et al. Effectiveness and limitations of hand hygiene promotion on decreasing healthcare-associated infections. PLoS One 2011; 6(11): e27163. doi: 10.1371/journal.pone.0027163
- Akyol A, Ulusoy H, Özen I. Handwashing: a simple, economical and effective method for preventing nosocomial infections in intensive care units. J Hosp Infect 2006; 62(4): 395–405. doi: 10.1016/j.jhin.2005.10.007
- Aboderin AO. Nosocomial infection and the challenges of control in developing countries. African J Clin Exp Microbiol 2010; 11(May): 102–10.
- Lenglet A, van Deursen B, Viana R, et al. Inclusion of real-time hand hygiene observation and feedback in a multimodal hand hygiene improvement strategy in low-resource settings. JAMA Netw Open 2019; 2(8): e199118. doi: 10.1001/jamanetworkopen.2019.9118
- Staines A, Vanderavero P, Duvillard B, et al. Sustained improvement in hand hygiene compliance using a multi-modal improvement programme at a Swiss multi-site regional hospital. J Hosp Infect 2018; 100(2): 176–82. doi: 10.1016/j.jhin.2018.04.010
- Santana SL, Furtado GHC, Coutinho AP, Medeiros EAS. Assessment of healthcare professionals’ adherence to hand hygiene after alcohol-based hand rub introduction at an intensive care unit in São Paulo, Brazil. Infect Control Hosp Epidemiol 2007; 28(3): 365–7. doi: 10.1086/510791
- World Health Organization. Guide to implementation: a guide to the implementation of the WHO multimodal hand hygiene improvement strategy. World Heal Assoc Press; 2009, pp. 1–48. Available from: https://www.who.int/gpsc/5may/Guide_to_Implementation.pdf?ua=1 [cited 27 July 2020].
- World Health Organization. Hand hygiene technical reference manual: to be used by health-care workers, trainers and observers of hand hygiene practices. Available from: https://apps.who.int/iris/handle/10665/44196 [cited 27 July 2020].
- McGuckin M, Waterman R, Govednik J. Hand hygiene compliance rates in the United States-a one-year multicenter collaboration using product/volume usage measurement and feedback. Am J Med Qual 2009; 24(3): 205–13. doi: 10.1177/1062860609332369
- Lambe KA, Lydon S, Madden C, et al. Hand hygiene compliance in the ICU: a systematic review. Crit Care Med 2020: 1251–7. doi: 10.1097/CCM.0000000000003868
- Murphy RA, Chua AC. Prevention of common healthcare-associated infections in humanitarian hospitals. Curr Opin Infect Dis 2016; 29(4): 381–7. doi: 10.1097/QCO.0000000000000285
- Alp E, Damani N. Healthcare-associated infections in intensive care units: epidemiology and infection control in low-to-middle income countries. J Infect Dev Ctries 2015; 9(10): 1040–5. doi: 10.3855/jidc.6832
- Al-Tawfiq JA, Abed MS, Al-Yami N, Birrer RB. Promoting and sustaining a hospital-wide, multifaceted hand hygiene program resulted in significant reduction in health care-associated infections. Am J Infect Control 2013; 41(6): 482–6. doi: 10.1016/j.ajic.2012.08.009
- Chun HK, Kim KM, Park HR. Effects of hand hygiene education and individual feedback on hand hygiene behaviour, MRSA acquisition rate and MRSA colonization pressure among intensive care unit nurses. Int J Nurs Pract 2015; 21(6): 709–15. doi: 10.1111/ijn.12288
- Thi Anh Thu L, Thi Hong Thoa V, Thi Van Trang D, et al. Cost-effectiveness of a hand hygiene program on health care-associated infections in intensive care patients at a tertiary care hospital in Vietnam. Am J Infect Control 2015; 43(12): e93–9. doi: 10.1016/j.ajic.2015.08.006
- Das Neves ZC, Tipple AF, Silva e Souza AC, Pereira MS, Melo Dde S, Ferreira LR. Hand hygiene: the impact of incentive strategies on adherence among healthcare workers from a newborn intensive care unit. Rev Lat Am Enfermagem 2006; 14(4): 546–52. doi: 10.1590/s0104-11692006000400012
- Duggan JM, Hensley S, Khuder S, Papadimos TJ, Jacobs L. Inverse correlation between level of professional education and rate of handwashing compliance in a teaching hospital. Infect Control Hosp Epidemiol 2008; 29(6): 534–8. doi: 10.1086/588164
- Maheshwari V, Kaore NCM, Ramnani VK, Gupta SK, Borle A, Rituja K. A study to assess knowledge and attitude regarding hand hygiene amongst residents and nursing staff in a tertiary health care setting of Bhopal city. J Clin Diagnostic Res 2014; 8(8): 4–7. doi: 10.7860/JCDR/2014/8510.4696
- Pittet D, Allegranzi B, Boyce J. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect Control Hosp Epidemiol 2009; 30(7): 611–22. doi: 10.1086/600379
- Allegranzi B, Storr J, Dziekan G, Leotsakos A, Donaldson L, Pittet D. The first global patient safety challenge “Clean Care is Safer Care”: from launch to current progress and achievements. J Hosp Infect 2007; 65: 115–23. doi: 10.1016/s0195-6701(07)60027-9