ORIGINAL ARTICLE
Spatial and temporal distribution of faecal indicators and multidrug resistant bacteria in a multiple-use freshwater lake: the case of Lake Hawassa, Ethiopia
Deresse Daka1*, Hunachew Beyene2 and Simachew Dires2
1School of Medical Laboratory Sciences, Hawassa University College of Medicine, Hawassa, Ethiopia; 2Department of Environmental Health, Hawassa University College of Medicine, Hawassa, Ethiopia
Abstract
Background: Aquatic environments close to cities are frequently used as sources for water and at the same time overloaded with a variety of pollutants either through direct or indirect discharges of untreated wastes and sewage. This condition is also worsened by the indiscriminate disposal of untreated wastes and sewage vigorously into used water. Sewage contaminated waters are known to carry microorganisms, some of which are pathogenic to humans.
Aim: The aim of this study was to assess the extent of temporal and spatial levels of microbial pollution and sources of pollution in Lake Hawassa.
Method: A cross-sectional study was conducted at Lake Hawassa, which was sampled twice during 2017. A total of 26 samples of lake water were collected from 14 stations using a boat. Entry points of incoming streams, waste receiving sites, and areas upstream of anthropogenic impact, recreational and bathing sites were considered. Microbiological characterisation was performed using selective media and basic biochemical tests. Antibiotic sensitivity was tested with different antibiotics using the Kirby-Bauer agar disk diffusion method.
Result: All samples were positive for pathogenic bacteria, including Gram-positive and Gram-negative bacteria. Enterobacteriaceae were the most common bacteria identified from the samples, including Escherichia coli, Salmonella spp, Shigella spp, Proteus spp and Gram-positive bacteria, such as Staphylococcus aureus. The predominant bacteria found in the samples include E. coli, which constituted 22/26 (84.6%) of the total samples, followed by Salmonella and Shigella spp. All bacterial isolates were resistant to penicillin and ampicillin. The Salmonella spp were sensitive only to norfloxacin and gentamicin.
Conclusion: A spatial variation with the occurrence of bacterial isolates has been observed. High concentrations and many different species were found in areas of human activities and in areas receiving direct pollutants from the city. This study revealed that multidrug resistant (MDR) pathogenic bacteria are found in Lake Hawassa. There is a possibility of outbreak of diseases associated with the isolated antibiotic-resistant pathogens for which the antibiotic resistance genes are transportable within aquatic bacterial communities. We recommend that the city administration take care of the municipal wastewater or effluents from healthcare facilities that enter the lake. It is also recommended that the government take steps to control anthropogenic activities near the water body.
Keywords: multidrug resistance; water microbiology; lakes; wastewater; Lake Hawassa; Ethopia
Citation: Int J Infect Control 2021, 17: 20428 – http://dx.doi.org/10.3396/ijic.v17.20428
Copyright: © 2021 Deresse Daka et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material for any purpose, even commercially, provided the original work is properly cited and states its license.
Received: 5 June 2020; Revised: 29 June 2020; Accepted: 18 December 2020; Published: 25 October 2021
Competing interests and funding: The authors declare that they have no competing interests.
The study was supported by Hawassa University. The support included payment for data collectors and purchase of materials and supplies required for the study. The support did not include designing of the study, analysis, and interpretation of data and manuscript preparation.
*Deresse Daka, School of Medical Laboratory Sciences, Hawassa University College of Medicine, P.O. Box 1560, Hawassa, Ethiopia. Email: drsdk200@gmail.com
Water bodies located near urban areas are often subject to receiving untreated and partially treated wastewater from a variety of point and non-point sources with diverse microbiological compositions. This poses risk to human health as a high density of microorganisms from diverse origins mix and may give rise to new antibiotic-resistant strains as a result of genetic exchange between the different microbes (1).
Several studies indicate that multidrug resistant (MDR) micro-organisms and genes responsible for antibiotic resistance are common in water bodies receiving different categories of wastes (2–5). Specifically, there is prevalence of MDR microbes in coastal water bodies as a result of uncontrolled application of antibiotics in our present-day society (6). The most important source of multidrug resistance in the environment appears to be the input of resistant bacteria from various sources, such as healthcare facilities for both human and veterinary purposes (7).
MDR microorganisms were isolated from effluents of the wastewater treatment plant (WWTP), which had the capacity of removing 99% bacteria (8). This indicates that the conventional water treatment plants, which are commonly used for municipal wastewater treatment, are not enough to remove them from the wastewater stream. Discharges from WWTPs may contribute to the release of antibiotic resistance genes into the environment (9–11).
Coastal regions receive wastewater from various categories of healthcare facilities with high composition of antibiotic residues. These residues are also present in domestic sewage when the residents take antibiotics to treat infections (12). These residues in the environment can select for antibiotic resistant bacteria that harbour mobile genetic elements, which can, in turn, be associated with resistance genes that are transferred to other indigenous microorganisms (13). For example, higher frequencies of Escherichia coli isolates possessing virulence and antimicrobial resistance genes were observed in an urban site located downstream of wastewater effluent outlets than in other examined sites, such as St. Clair River and Detroit River areas, USA (4).
A study on water quality analysis in South Africa revealed that bacterial pathogens, which were resistant to several classes of antibiotics, were isolated from a drinking water distribution system (14). This implies that there might be a direct risk of acquiring infections related to MDR microbes. These microbes were also identified from surface water samples near rural settlements in Mexico (3).
The freshwater Lake Hawassa has no known outlet of water and is located adjacent to Hawassa City, with a population of more than 200,000 people. There is no municipal wastewater treatment system in place, and there are several public and private healthcare facilities, factories, hotels and other business centres in the city. The lake serves as a centre for recreational boating, bathing, clothes washing, irrigation, drinking, and as a sink for disposal of domestic and industrial wastes.
Reports show that in the catchment area of Lake Hawassa, there has been a reduction in natural land cover mainly due to the expansion of agricultural lands and urban areas, which resulted in environmental degradation and increase in the average inflow of waste into the lake. The quality of the water in the lake is deteriorating due to the entry of pollutants from the nearby agricultural lands and the city (15–17).
Following the increased migration from rural areas and nearby towns, there has been extensive land development near Lake Hawassa, with attendant contaminated runoff being a significant contribution to contamination at Lake Hawassa. These activities have resulted in the lake becoming a reservoir for MDR microorganisms, which can contain transmissible genes and also exhibit better survival potential for an extended time period under a wide range of changing environmental conditions.
Multidrug resistance is defined as non-susceptibility to at least one agent in three or more antimicrobial categories (18). Studies related to MDR pathogens in Ethiopian water bodies are lacking. The current study aimed to determine the prevalence of MDR pathogens, and their spatial and temporal variations across Lake Hawassa.
Methods
Description of the study area
Lake Hawassa is located at the western shore of Hawassa City, the capital city of South Nation Nationality and Peoples Region. It is situated 275 km away from Addis Ababa. Lake Hawassa (Lat: 60 33’–70 33’ N; Long: 380 22’–380 29’ E) is located at an altitude of 1,680 m in the Ethiopian Rift Valley (Fig. 1). The region is characterised by a dry, sub-humid climate, with a monthly mean minimum air temperature of 7.0–13.7°C and a maximum air temperature of 23.0–32.0°C. Surface water temperature of the lake ranged from 17.8 to 25.2°C in 1987–1988 (19).
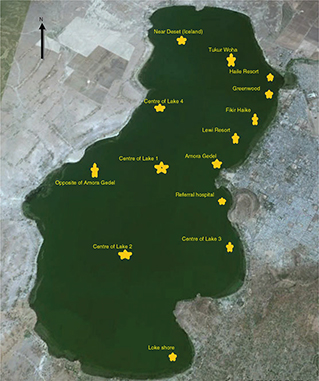
Fig. 1. Water sampling locations.
Source: Hawassa Administrative office, 2017.
The Rift Valley climate is characterised by a 4-month dry season (November–February) and an 8-month rainy season (March–October) (20). Rainfall is subject to variability according to altitude. In general, the plateaus over 2,500 m above sea level (masl) receive 1,400–1,800 mm/year, mid-altitude regions (600–2,500 masl) receive 1,000–1,400 mm/year and coastal lowlands receive less than 200 mm/year (21).
Lake Hawassa lacks any obvious outlet but is fed by a small river, the Tikur Woha. There is a regular annual fluctuation in water level. In the dry season, the water level decreases to more than 1 m compared with rainy season levels (19). The water levels in lake fluctuate considerably over the years in response to variations in rainfall and evaporation. The surface area is 90 km2, the catchment area is 1,250 km2, the max depth is 22 m and the mean depth is 11 m (22).
Lake Hawassa is the backbone of Hawassa City and the most important place for fishing activities in the region for both local and national markets. It is also used for cultivation of vegetables and recreational purposes, such as swimming and washing of clothes. The lake has been receiving a huge volume of wastewater from various small- to large-scale industries, healthcare institutions, hotels and flood water from the city.
Sampling and sample collection
The sampling was conducted twice during the year 2017. A total of 26 samples were collected from May 13–15 to November 12–15, 2017. Twelve and 14 water samples were collected in the first and second rounds, respectively, from Lake Hawassa, and were analysed in the Hawassa University Referral Hospital laboratory.
Sampling equipment and procedure
Fourteen samples of lake water at 14 stations were collected in clean and sterile 250 mL brown glass bottles. The bottles filled with sample water were then packed in Whirl-Pak® collection bags and transported to microbiology laboratory (23). Entry points for incoming streams, waste receiving sites, and areas upstream of anthropogenic impact, recreational and bathing sites were considered (Fig. 1). Samples were collected in 250 mL sterile bottles (23), kept approximately 10 cm beneath the surface of the water until full. The pre-labelled containers were sealed and kept on ice until they were analysed in the laboratory. MDR is non-susceptible to at least one agent in three or more antimicrobial categories (18).
Analysis
Microbiological analyses were carried out using mannitol salt agar, MacConkey agar media, nutrient agar, peptone water and blood agar. Biochemical tests, including urea test, triple sugar iron (TSI), lysine agar, indole test and hydrogen sulfide tests, were conducted (Oxoid Basingstoke, UK). Antibiotic sensitivity tests were also performed using different antibiotics, including penicillin (10 µg), ampicillin (10 µg), ceftriaxone (30 µg), gentamicin (10 µg) and vancomycin (30 µg), using the Kirby-Bauer agar disk diffusion method (24). These groups of antibiotics were chosen because they were the most commonly prescribed drugs in the local hospital.
The total colony count (TCC) of bacteria was determined using the pour plate method (25) with nutrient agar (Oxoid Basingstoke, UK). The water samples were serially diluted, and the plates were adequately diluted in duplicate. The bacteria were characterised based on the taxonomic system and descriptions (26).
Test for faecal coliforms and identification of E. coli
The three-tube technique with lactose broth (27) was used to identify coliforms to get the most probable number of coliform bacteria.
Faecal (thermotolerant) coliforms compose a subset of total coliforms. These bacteria also grow and ferment lactose with production of gas and acid at 44.5 ± 0.2°C within the first 48 h of incubation on Brilliant Green Bile Broth (28). The contents of these tubes were inoculated on eosin-methylene blue (EMB) agar and then incubated. The growth of E. coli on EMB plates was identified based on the biochemical, cultural and morphological characteristics (23), and the number of E. coli was determined.
Results
All samples tested were positive for pathogenic bacteria, including both Gram-positive and Gram-negative bacteria. The most common bacteria identified from the samples include Enterobacteriaceae: E. coli, Salmonella spp, Shigella spp, Proteus mirabilis and Gram-positive bacteria, such as S. aureus (Table 1). The second round of sampling showed the highest bacterial concentration in the lake when comparing the two sampling times.
The most common pathogenic bacteria included Gram-negative bacteria, all belonging to Enterobacteriaceae, and all samples were contaminated with different bacterial species. The most prevalent bacteria included Staphylococcus spp, Salmonella spp, Shigella spp, Proteus mirabilis and E. coli.
The highest colony count of the study was reported at Tikur Woha, Amora Gedel and Greenwood inlet areas (Fig. 1 and Table 2).
Most organisms identified from Lake Hawassa were MDR. Salmonella spp were only sensitive to norfloxacin and gentamicin (Tables 3 and 4).
Discussion
The results of this study reveal the prevalence of MDR bacteria in Lake Hawassa, which indicate that the lake water is polluted with antibiotic residues from the wastewater of the adjacent city and nearby agricultural lands. Most organisms identified were MDR. The presence of antibiotic-resistant bacteria in a given environment could be an indication that the area is polluted with antibiotics (29). Environmental antibiotic concentrations may exert selective pressure on environmental bacteria and may also foster the transfer of resistance genes, helping to create the ‘resistome’ mixing pot of genetic antimicrobial resistance traits (30). Antibiotic resistance of faecal bacteria in surface waters has been studied by various researchers from different types of surface waters, including rivers (4, 13, 31–33).
The most reliable indicator of faecal contamination in water is commonly the presence of coliforms and E. coli originating from human and animal intestines. Both Enterobacter spp and E. coli were present in the samples collected. The results of this study reveal the widespread distribution of E. coli in aquatic ecosystems. The potential public health threat of E. coli pathotypes was found to originate from municipal wastewater sources (4). This may lead to antibiotic-resistant bacteria that harbour mobile genetic elements of bacteria. The mobile genetic elements can consecutively be associated with resistant genes that are transferred to other resident microorganisms in the soil, water and gut ecosystems (13).
The main reasons for lake contamination by resistant bacteria may be improper and unnecessary use of antimicrobial drugs by human beings and animals (34) and improper disposal of sewage from various sources of the city and the nearby agricultural land. The present assessment of the microbial population for the antibiotic resistance profile showed a high proportion of strains resistant to penicillin. This indicates that these medicines have been extensively used in the area, and the available treatment plants might not be removing either microorganisms or antibiotics properly.
The research studies worldwide have shown that antibiotic resistance is a common characteristic of freshwater bodies. Bacteria isolated from water of the Łabędzi Pond in Poland are characterised by large differences in the level of resistance to antibiotics. Bacteria inhabiting the waters of the Łabędzi Pond were found to be characterised by multi-antibiotic resistance (35).
Another study conducted in Emajõgi river in Estonia showed that isolates of several antibiotic-resistant species were distributed in the river (36). A similar study also revealed that concentrations of E. coli were generally high along the mainstream and tributaries, although a higher level of resistance in E. coli isolates at downstream sites was expected than the upstream sites due to high levels of E. coli in the downstream samples (32).
The study was conducted in different locations of the lake, considering that specific sites might receive wastes directly while others do not. Accordingly, we have found that there have been variations in MDR patterns among sampling locations. Most of the MDR was observed in sampling locations where there was much human activity, such as Fikir Haike. This indicates that extensive, uncontrolled human activity near the lake leads to contamination of the site. However, in a similar study, there was no significant difference in antibiotic resistance profiles observed among the sites or between seasons. However, the only difference found was for resistance in E. coli strains to streptomycin, which was affected by the wastewater treatment plant effluent (32).
In this study, the variations of numbers in colony forming unit of isolates have been found across seasons (Table 2). A larger number of E. coli was found in the samples taken during November, when the lake was maximum full. This might be due to the entry of runoff water with many pathogens as a result of heavy rain prior to sampling. The lake water is being used for irrigation of small-scale agricultural farms adjacent to the lake. Using water containing bacteria having high antibiotic resistance levels is a potential public health risk, as well as the possibility of transmitting antibiotic resistance to bacterial populations in the environment (32, 37).
Conclusion
This research study demonstrates and confirms that discharges of wastewater from different inlets may increase the prevalence of pathogenic bacteria. The selected indicators may give better information on the development of effective antibiotic resistance controls for protecting surface water quality and public health. Future studies will discuss the effects of outlet and inlet wastewater on Lake Hawassa. The organisms identified in this study were MDR. Antibiotics, such as doxycycline and ampicillin, were not effective on all identified bacteria, except on few species of E. coli. Salmonella species were only sensitive to norfloxacin and gentamicin.
Recommendation
To maintain the sustainable use of Lake Hawassa, the following recommendations have been made to various concerned organisations, especially city administration:
- The possible entry of untreated wastes in the lake from various point sources needs to be verified and monitored.
- The city administration needs to implement proper solid liquid waste management strategies to avoid the entry of drug residues and microbes in the lake during flooding.
- Installations built near the lake need to have the appropriate liquid waste treatment system.
- City administration needs to put a strategy in place to monitor the activities of those installations.
Acknowledgement
This research work was funded by Hawassa University. The authors thank the sample collector and the city administrators for supporting the study.
Consent for publication
Not applicable.
Ethics approval
The study has been conducted after approved by the IRB (Institutional Review Board) of Hawassa University College of Medicine and Health Sciences. Verbal informed consent was obtained from the Hawassa city municipality. The purpose of the study and the confidential nature of the study were discussed with municipality.
Authors’ contribution
DD collected samples and did all laboratory work, analysis and writing up. HB writing up and analysis and SD writing up and analysis.
References
- Flor-Yazmín RC, Josée H, Adriana-Cecila MF, Abraham LM, Alma-Lilián GB, Francisco-Javier AG. Antimicrobial resistance: the role of aquatic environments Int J Curr Res Accad Rev 2014; 2(7): 231–46.
- Megan B, Ellen J, David M. Occurrence of multiple antibiotic resistant bacteria in aquatic environments in central Minnesota. Am J Undergrad Res 2015; 12(3): 19–35. doi: 10.33697/ajur.2015.012
- Delgado-Gardea MC, Tamez-Guerra P, Gomez-Flores R, Zavala-Díaz de la Serna FJ, Eroza-de la Vega G, Nevárez-Moorillón GV. Multidrug-resistant bacteria isolated from surface water in Bassaseachic Falls National Park, Mexico. Int J Environ Res Public Health 2016; 13(6): 597. doi: 10.3390/ijerph13060597
- Hamelin K, Bruant G, El-Shaarawi A, Hill S, Edge TA, Fairbrother J. Occurrence of virulence and antimicrobial resistance genes in Escherichia coli isolates from different aquatic ecosystems within the St. Clair River and Detroit River areas. Appl Environ Microbiol 2007; 73(2): 477–84. doi: 10.1128/AEM.01445-06
- Ha NY, Seo JK, Joo-Hyon K, Young-Sik H, Sung MC, Seung WL. Occurrence of antibiotic resistant E. coli in surface water: a study in a typical urban watershed, Korea. Water Pract Technol 2010; 5: 1–10. doi: 10.2166/wpt.2010.056
- Aayushi M, Sunil B, Rutuja D, Gajbhiye SN, Syed G. Occurrence and distribution of multiple antibiotic-resistant bacteria of Enterobacteriaceae family in waters of Veraval coast, India. Environ Exp Biol 2014; 12: 43–50.
- Jalal K, Akbar JB, Kamaruzzaman BY, Kathiresan K. Emergence of antibiotic resistant bacteria from coastal environment – a review. In: Pana M, ed. Antibiotic resistant bacteria – a continuous challenge in the new millennium. Croatia, Rijeka: InTech; 2012, p. 3–14.
- Korzeniewska E, Korzeniewska A, Harnisz M. Antibiotic resistant Escherichia coli in hospital and municipal sewage and their emission to the environment. Ecotoxicol Environ Saf 2013; 91: 96–102. doi: 10.1016/j.ecoenv.2013.01.014
- Conte D, Palmeiro JK, Keite da Silva Nogueira, Rosa de Lima TM, Cardoso MA, Pontarolo R, et al. Characterization of CTX-M enzymes, quinolone resistance determinants, and antimicrobial residues from hospital sewage, wastewater treatment plant, and river water. Ecotoxicol Environ Saf 2017; 136: 62–9. doi: 10.1016/j.ecoenv.2016.10.031
- Elisabet M, Juan J, Jose LB. Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PLoS One 2013; 8(10): e78906. doi: 10.1371/journal.pone.0078906
- Silva J, Castillo G, Callejas L, López H, Olmos J. Frequency of transferable multiple antibiotic resistance amongst coliform bacteria isolated from a treated sewage effluent in Antofagasta, Chile. Electron J Biotechnol 2006; 9: 534–540. doi: 10.2225/vol9-issue5-fulltext-7
- Madikizela ML, Tavengwa NT, Chimuka L. Status of pharmaceuticals in African water bodies: occurrence, removal and analytical methods. J Environ Manage 2017; 193: 211–20. doi: 10.1016/j.jenvman.2017.02.022
- Hong PY, AL-Jassim N, Ansari M, Mackie R. Environmental and public health implications of water reuse: antibiotics, antibiotic resistant bacteria, and antibiotic resistance genes. Antibiotics 2013; 2(3): 367–99. doi: 10.3390/antibiotics2030367
- Mulamattathil SG, Bezuidenhout C, Mbewe M, Ateba CN. Isolation of environmental bacteria from surface and drinking water in Mafikeng, South Africa, and characterization using their antibiotic resistance profiles. J Pathog 2014; 2014: 11. doi: 10.1155/2014/371208
- Daniel S, Yonas M. Assessing the effect of land use change on the hydraulic regime of Lake Awassa. Nile Basin Water Sci Eng J 2010; 3(2): 110–8.
- Nigatu W, Øystein B, Havard T. GIS based mapping of land cover changes utilizing multi-temporal remotely sensed image data in Lake Hawassa Watershed, Ethiopia. Environ Monit Assess 2014; 186: 1765–80. doi: 10.1007/s10661-013-3491-x
- Gessesse D, Carl C. Forest decline and its causes in the south-central rift valley of Ethiopia: human impact over one hundred year perspective. Ambio 2008; 37(4): 263–71. doi: 10.1579/0044-7447(2008)37[263:FDAICI]2.0.CO;2
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas M E, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. CMI 2012; 18(3): 268–81. doi: 10.1111/j.1469-0691.2011.03570.x
- Admassu D, Ahlgren I. Growth of juvenile tilapia Oreochromis niloticus L. from Lakes Zwai, Langano and Chamo (Ethiopian Rift Valley) based on otolith microincrement analysis. Ecol Freshw Fish 2000; 9: 127–37. doi: 10.1111/j.1600-0633.2000.eff090301.x
- Tudorancea C, Zinabu GM, Dadebo E. Limnology in Ethiopia. Limnol Dev Ctries 1999; 2: 63–118.
- Breuil C. Review on the fisheries and aquaculture sector: Ethiopia. FAO Fisheries Circular. No 890. Rome: FAO; 1995, 29p.
- Kebede E, Gebre-Mariam Z, Ahlgren I. The Ethiopian Rift valley lakes: chemical characteristics of a salinity-alkalinity series. Hydrobiologia 1994; 288: 1–12. doi: 10.1007/BF00006801
- APHA. Compendium of methods for the microbiological examination of foods. Vanderzant C, Splittstoesser DF, eds. 3rd edn. Washington, DC: APHA; 1992. Inc. ISBN 0-87553173-3.
- CLSI. Performance standards for antimicrobial disk susceptibility tests. 13th Edition. CLSI Standard M-02. Wayne PA: Clinical and Laboratory Standards Institute; 2018.
- Nwachukwu S. Enhanced rehabilitation of tropical aquatic environment polluted with crude petroleum using Candida utilis. J Environ Biol 2000; 21: 241–50.
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s manuel of determinative bacteriology. 9th edn. Baltimore, MD: Williams and Wilkins; 1994, p. 787.
- Bakare AA, Lateef A, Amuda OS, Afolabi RO. The Aquatic toxicity and characterization of chemical and microbiological constituents of water samples from Oba River, Odo-oba, Nigeria. Asian J Microbiol Biotechnol Environ Sci 2003; 5: 11–7.
- Coyne MS, Howell JM. The fecal coliform/fecal streptococci ratio (FC/FS) and water quality in the bluegrass region of Kentucky. Soil Sci News Views 1994; 15.
- Gunaseelan C, Ruban P. Antibiotic resistance of bacteria from Krishna Godavari Basin, Bay of Bengal, India. Environ Exp Biol 2011; 9: 133–6.
- WHO, Future trends in veterinary public health. World Health Organ Tech Rep Ser 2002; 907: 1–85.
- Abhirosh C, Suson PS, Thomas AP, Mohamed H, Asit M. Survival of multi-drug resistant enteropathogenic Escherichia coli and Salmonella paratyphi in Vembanadu lake as a function of saltwater barrier along southwest coast of India. J Water Health 2013; 11(2): 324–32. doi: 10.2166/wh.2013.221
- Ha-Na Y, Seo-Jin Ki, Joo-Hyon K, Young-Sik H, Sung MC, Seung WL, et al. Occurrence of antibiotic resistant E. coli in surface water: a study in a typical urban watershed, Korea. Water Pract Technol 2010; 5(10): 1–10.
- Qian Y, Dongmei Y, Yuke P, Ying L, Lin X. Occurrence and distribution of antibiotic-resistant bacteria and transfer of resistance genes in lake Taihu. Microbes Environ 2013; 28(4): 479–86. doi: 10.1264/jsme2.ME13098
- Al-Bahry S, Mahmoud I, Elshfie A, Al-Harthy A, Al-Ghafri S, Al-Amri Iet al. Bacterial flora and antibiotic resistance from eggs of green turtles Chelonia myans: an indication of polluted effluents. Mar Pollut Bull 2009; 58(5): 720–5. doi: 10.1016/j.marpolbul.2008.12.018
- Zbigniew M, Piotr S. Frequency of antibiotic resistance in bacteria inhabiting water of downtown pond. Słupsk, Poland: Baltic Coastal Zone; 2009, p. 135–46.
- Voolaid V, Joers A, Kisand V, Tenson T. Co-occurrence of resistance to different antibiotics among aquatic bacteria. BMC Microbiol 2012; 12: 225. doi: 10.1186/1471-2180-12-225
- Juan S, Gabriela C, Lorena C, Héctor L, Janet O. Frequency of transferable multiple antibiotic resistance amongst coliform bacteria isolated from a treated sewage effluent in Antofagasta, Chile. Electron J Biotechnol 2006; 9(5): 533–40. doi: 10.2225/vol9-issue5-fulltext-7